Formula C12O12Ru3 Density 2.48 g/cm³ Appearance orange solid | Molar mass 639.33 g/mol Melting point 224 °C | |
 | ||
Related compounds | ||
Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.
Contents
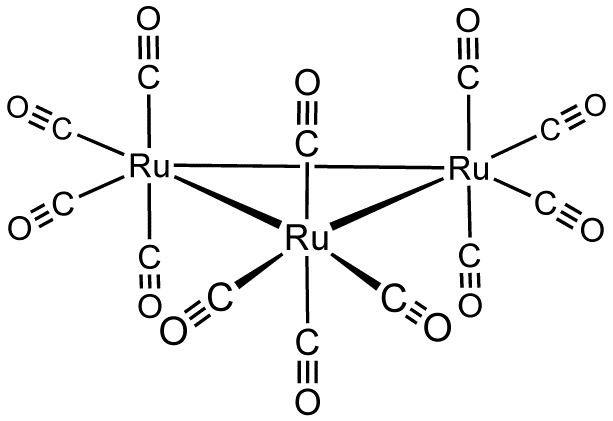
Structure and synthesis
The cluster has D3h symmetry, consisting of an equilateral triangle of Ru atoms, each of which bears two axial and two equatorial CO ligands. Os3(CO)12 has the same structure, whereas Fe3(CO)12 is different, with two bridging CO ligands, resulting in C2v symmetry.
Ru3(CO)12 is prepared by treating solutions of ruthenium trichloride with carbon monoxide, usually under high pressure. The stoichiometry of the reaction is uncertain, one possibility being the following:
6 RuCl3 + 33 CO + 18 CH3OH → 2 Ru3(CO)12 + 9 CO(OCH3)2 + 18 HClReactions
The chemical properties of Ru3(CO)12 have been widely studied, and the cluster has been converted to hundreds of derivatives. High pressures of CO convert the cluster to the monomeric ruthenium pentacarbonyl, which reverts to the parent cluster upon standing.
Ru3(CO)12 + 3 CO ⇌ 3 Ru(CO)5 Keq = 3.3 x 10−7 mol dm−3 at room temperatureThe instability of Ru(CO)5 contrasts with the robustness of the corresponding Fe(CO)5. The condensation of Ru(CO)5 into Ru3(CO)12 proceeds via initial, rate-limiting loss of CO to give the unstable, coordinatively unsaturated species Ru(CO)4. This tetracarbonyl binds Ru(CO)5, initiating the condensation.
Upon warming under a pressure of hydrogen, Ru3(CO)12 converts to the tetrahedral cluster H4Ru4(CO)12. Ru3(CO)12 undergoes substitution reactions with Lewis bases:
Ru3(CO)12 + n L → Ru3(CO)12-nLn + n CO (n = 1, 2, or 3)where L is a tertiary phosphine or an isocyanide.
Ru-carbido clusters
At high temperatures, Ru3(CO)12 converts to a series of clusters that contain interstitial carbido ligands. These include Ru6C(CO)17 and Ru5C(CO)15. Anionic carbido clusters are also known, including [Ru5C(CO)14]2− and the bioctahedral cluster [Ru10C2(CO)24]2−. Ru3(CO)12 -derived carbido compounds have been used to synthesize nanoparticles for catalysis. These particles consist of 6-7 atoms and thus are all surface, resulting in extraordinary activity.
