 | ||
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as dinitrogen and carbon monoxide are also triple bonded. In skeletal formula the triple bond is drawn as three parallel lines (≡) between the two connected atoms.
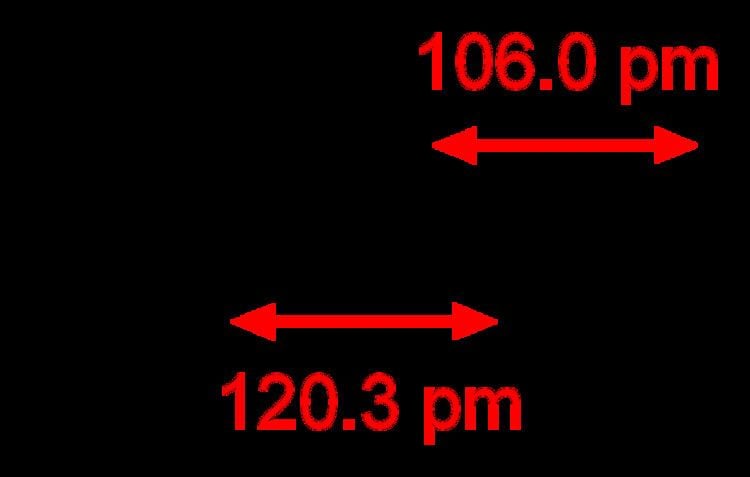
Triple bonds are stronger and shorter than the equivalent single bonds or double bonds, with a bond order of three.
Bonding
The types of bonding can be explained through in terms of orbital hybridization. In the case of acetylene each carbon atom has two sp orbitals and two p-orbitals. The two sp orbitals are linear with 180° angles and occupy the x-axis (cartesian coordinate system). The p-orbitals are perpendicular on the y-axis and the z-axis. When the carbon atoms approach each other the sp orbitals overlap to form a sp-sp sigma bond. At the same time the pz-orbitals approach and together they form a pz-pz pi-bond. Likewise, the other pair of py-orbitals form a py-py pi-bond. The result is formation of one sigma bond and two pi bonds.
In the bent bond model the triple bond can also formed by the overlapping of three sp3 lobes without the need to invoke a pi-bond.
