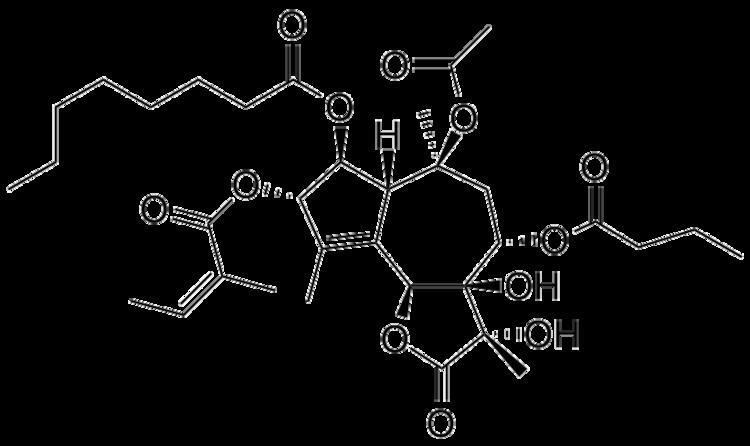
Molar mass 650.76 g/mol | ||
 | ||
2apb and thapsigargin on hacat cells
Thapsigargin is non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA). Structurally, thapsigargin is classified as a sesquiterpene lactone, and is extracted from a plant, Thapsia garganica. It is a tumor promoter in mammalian cells.
Contents
- 2apb and thapsigargin on hacat cells
- csa and thapsigargin on hacat cells
- Biosynthesis
- Research
- References
Thapsigargin raises cytosolic (intracellular) calcium concentration by blocking the ability of the cell to pump calcium into the sarcoplasmic and endoplasmic reticula. Store-depletion can secondarily activate plasma membrane calcium channels, allowing an influx of calcium into the cytosol.
Thapsigargin specifically inhibits the fusion of autophagosomes with lysosomes; the last step in the autophagic process. The inhibition of the autophagic process in turn induces stress on the endoplasmic reticulum which ultimately leads to cellular death.
Thapsigargin is useful in experimentation examining the impacts of increasing cytosolic calcium concentrations.
csa and thapsigargin on hacat cells
Biosynthesis
The complete biosynthesis of thapsigargin has yet to be elucidated. A proposed biosynthesis starts with the farnesyl pyrophosphate. The first step is controlled by the enzyme germacrene B synthase. In the second step, the C(8) position is easily activated for an allylic oxidation due to the position of the double bond. The next step is the addition of the acyloxy moiety by a P450 acetyltransferase; which is a well known reaction for the synthesis of the diterpene, taxol. In the third step, the lactone ring is formed by a cytochrome P450 enzyme using NADP+. With the butyloxy group on the C(8), the formation will only generate the 6,12-lactone ring. The fourth step is an epoxidation that initiates the last step of the base guaianolide formation. In the fifth step, a P450 enzyme closes the 5 + 7 guaianolide structure. The ring closing is important, because it will proceed via 1,10 - epoxidation in order to retain the 4,5 - double bond needed in thapsigargin. It is not known whether the secondary modifications to the guaianolide occur before, or after the formation of thapsigargin, but will need to be considered when elucidating the true biosynthesis. It should also be noted, that several of these enzymes are P450s, therefore oxygen and NADPH are likely crucial to this biosynthesis as well as other cofactors such as Mg2+ and Mn2+ may be needed.
Research
Since inhibition of SERCA is a mechanism of action that has been used to target solid tumors, thapsigargin has attracted research interest. A prodrug of thapsigargin, mipsagargin, is currently undergoing clinical trials for the treatment of glioblastoma.
The biological activity has also attracted research into the laboratory synthesis of thapsigargin. To date, two distinct syntheses have been reported, one by Steven V. Ley and one by Phil Baran.
