Related compounds | Formula Na4P2O7 | |
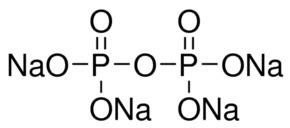 | ||
Appearance Colorless or white crystals | ||
Tetrasodium pyrophosphate
Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is a colorless transparent crystalline chemical compound with the formula Na4P2O7. It is a salt composed of pyrophosphate and sodium ions. Toxicity is approximately twice that of table salt when ingested orally. There is also a hydrated form, Na4P2O7 · 10(H2O).
Contents
Tetrasodium pyrophosphate is used as a buffering agent, an emulsifier, a dispersing agent, and a thickening agent, and is often used as a food additive. Common foods containing tetrasodium pyrophosphate include chicken nuggets, marshmallows, pudding, crab meat, imitation crab, canned tuna, and soy-based meat alternatives and cat foods and cat treats where it is used as a palatability enhancer.
In toothpaste and dental floss, tetrasodium pyrophosphate acts as a tartar control agent, serving to remove calcium and magnesium from saliva and thus preventing them from being deposited on teeth. Tetrasodium pyrophosphate is used in commercial dental rinses before brushing to aid in plaque reduction.
Tetrasodium pyrophosphate is sometimes used in household detergents to prevent similar deposition on clothing, but due to its phosphate content it causes eutrophication of water, promoting algae growth.
Tetrasodium pyrophosphate
Production
Tetrasodium pyrophosphate is produced by the reaction of furnace-grade phosphoric acid with sodium carbonate to form disodium phosphate, which is then heated to 450 °C to form tetrasodium pyrophosphate.
Alternatively, it can be created by the molecular dehydration of dibasic sodium phosphate at 500 °C (932 °F).
