Formula C12O12Rh4 Appearance Red crystals | Molar mass 747.743 g/mol | |
 | ||
Related compounds | ||
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest stable binary rhodium carbonyl. It is used as a catalyst in organic synthesis.
Contents
Structure, synthesis, reactions
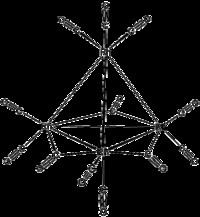
The structure of Rh4(CO)12 is described by a tetrahedral array of four Rh atoms with nine terminal CO ligands and three bridging CO ligands. The structure can be expressed as Rh4(CO)9(µ-CO)3.
It is prepared by treatment of an aqueous solution of rhodium trichloride with activated copper metal under an atmosphere of CO.
4 RhCl3(H2O)3 + 8 Cu + 22 CO → Rh4(CO)12 + 2 CO2 + 8 Cu(CO)Cl + 4 HCl + 10 H2OAlternatively, the compound can be prepared by treatment of a methanolic solution of RhCl3(H2O)3 with CO to afford H[RhCl2(CO)2], followed by carbonylation in the presence of sodium citrate.
The cluster undergoes thermal substitution with phosphorus ligands:
Rh4(CO)12-n + n L → Rh4(CO)12-nLn + n CORelated metal carbonyls
Because of their relevance to hydroformylation catalysis, the metal carbonyls has been systematically studied to a high degree. The instability of Rh2(CO)8 has been a source of curiosity. The analogous binary carbonyl of cobalt, Co2(CO)8, is well known. Solutions of Rh4(CO)12 under high pressures of CO convert to the dirhodium compound:
Rh4(CO)12 + 4 CO → 2 Rh2(CO)8Unlike Co2(CO)8, the main isomer of Rh2(CO)8 features only terminal CO ligands. The relative instability of Rh2(CO)8 is analogous to the tendency of Ru(CO)5 to convert to Ru3(CO)12.
