Formula H4BO4- | Molar mass 78.84 g/mol | |
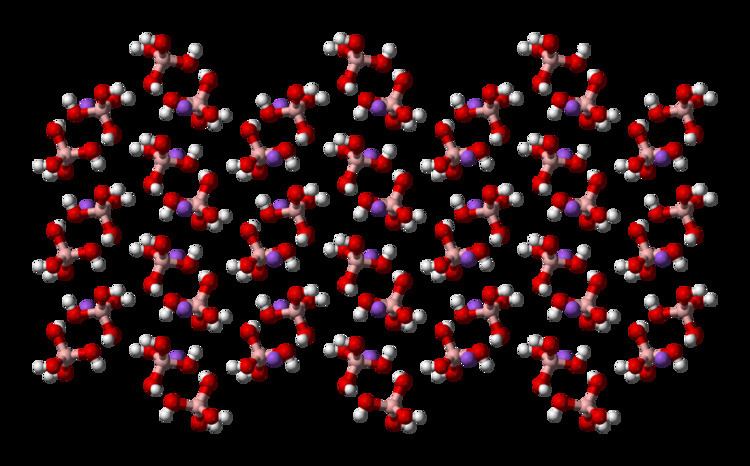 | ||
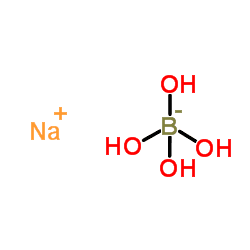
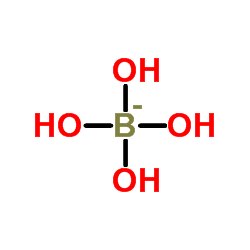
Tetrahydroxyborate (systematically named tetrahydroxyboranuide and tetrahydroxidoborate(1−)) is an inorganic anion with the chemical formula [B(OH)
4]−
(also written as B(OH)−
4 or BH
4O−
4). It contributes no colour to tetrahydroxyborate salts. It is found in the mineral hexahydroborite, Ca(B(OH)4)2·2H2O (originally formulated CaB2O4.6H2O). It is classified as a weak base.
Contents
Basicity
Tetrahydroxyborate can assimilate a proton into the anion by recombination:
B(OH)−4 + H+
→ B(OH)
3 + H
2O

Because of this capture of a proton (H+
), tetrahydroxyborate has Arrhenius-basic character. Its protonation product is boric acid. In aqueous solution, most tetrahydroxyborate ions are undissociated.
4
3 + OH−
Structure
It is a boron oxoanion with a tetrahedral geometry. It is isoelectronic with the hypothetical compound orthocarbonic acid.
Chemical reactions
Tetrahydroxyborate undergoes the typical chemical reactions of a hydroxyborate. Upon treatment with a standard acid, it converts to boric acid and a metal salt. Oxidation of tetrahydroxyborate gives perborate. When heated to a high temperature, tetrahydroxyborate salts decompose to produce metaborate salts and water, or to produce boric acid and a metal hydroxide:
n [B(OH)4]− → (BO−2)n + 2n H2O[B(OH)4]− → B(OH)3 + HO−
Production
Tetrahydroxyborate is produced by boric acid alkalinisation. In this process boric acid and hydroxide anions react to produce tetrahydroxyborate according to the following reaction:
B(OH)3 + HO− → [B(OH)4]−This process also involves aquatrihydroxyboron as an intermediate, and occurs in two steps. No catalyst is needed for alkalinisation (step 2).
- B(OH)3 + H2O → [B(OH)3H2O]
- [B(OH)3H2O] + HO− → [B(OH)4]− + H2O
Catalytic amounts of water are used for the process. By altering the process conditions, other borate anions may also be produced on the same production site.
Uses
Tetrahydroxyborate can be used as a cross-link in polymers.
Occurrence
The tetrahydroxyborate anion is found in Na[B(OH)4], Na2[B(OH)4]Cl and CuII[B(OH)4]Cl.
