Molar mass 322.3 g/mol Appearance Pale-yellow liquid | Density 1.2 g/cm³ | |
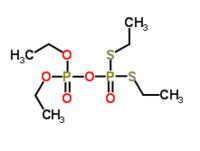 | ||
Tetraethyl dithiopyrophosphate (also known as TEDP or Sulfotepp) is a highly toxic chemical compound with the chemical formula C8H20O5P2S2. The chemical is a mobile oil that is pale yellow-colored and smells like garlic. It is primarily used as an insecticide, but does not damage plants significantly.
Contents
Properties
When heated to a temperature that is high enough for tetraethyl dithiopyrophosphate to decompose, it gives off fumes of phosphorus and sulfur oxides, which are highly toxic. It can explode if containers of it are heated, and it can burn, although it does not do so easily. The chemical can also polymerize explosively. The chemical also reacts to form toxic and flammable gases in the presence of hydrides and other reducing agents. It is able to corrode iron. When it does this, it can release hydrogen gas. The chemical has a specific gravity of 1.196 at 77 °F (25 °C) and its vapor density is 13.17 grams per liter at 25 °C (77 °F). Its melting point is 88 °C (190 °F) and its boiling point is between 272 °F (133 °C) and 282 °F (139 °C) at 2mm Hg. The chemical's sorption coefficient is 2.87 Log L/kg. Its Henry's Law constant is 0.000174594 at 20 °C (68 °F). Its octanol-water partition coefficient is 3.9804 Log L/kg. Tetraethyl dithiopyrophosphate's diffusion coefficient in air is 0.015 cm2 per second and its diffusion coefficient in water is 0.0000055 cm2.
Tetraethyl dipyrophosphate's flash point is 178.317 °C (352.971 °F) and its enthalpy of vaporization is 59.395 kilojoules per mole. Its surface tension is 42.9070014953613 dynes per centimeter. The chemical has no Rule of 5 violations. Its diffusivity in water is 0.63 × 10−5 cm2 per second. It is miscible with a large number of organic solvents, including methyl chloride and acetone and its solubility in water is 30 milligrams per liter at 20 °C (68 °F).
The alkaline and neutral hydrolysis of tetraethyl dithiopyrophosphate results in the release of ethanol, phosphoric acid, and hydrogen sulfide.
Production
Tetraethyl dithiopyrophosphate is produced in a manner similar to hexaethyl tetraphosphate.
Applications
Tetraethyl dithiopyrophosphate has applications as an insecticide, mitocide, and acaricide. However, because it does not leave behind a residue, it is less effective at these roles than DDT. However, it is about as effective as the insecticide parathion. Its use is restricted to greenhouses and ornamental plants. When the chemical is used as an insecticide, it is in the form of an impregnated smoke fumigant.
Biological role and toxicity
It is probable that less than five milligrams per kilogram of tetraethyl dithiopyrophosphate administered orally to a human will be lethal. This equates to fewer than seven drops of the chemical killing a 150 pounds (68 kg) human. The chemical is an inhibitor of cholinesterase.
If a human's exposure to tetraethyl dithiopyrophosphate is acute, then the resulting symptoms can include sweating, dizziness, contracted pupils, headaches, severe weakness, muscle spasms, seizures, and comas. Other possible symptoms from exposure to the chemical include confusion and psychosis, abdominal pain, nausea and vomiting, anorexia, and diarrhea and fecal incontinence. Hypotension and hypertension both occur when humans are exposed to tetraethyl dithiopyrophosphate. The heart rate decreases when the chemical is exposed to the skin, but increases when exposed orally. Damage to the respiratory system caused by exposure to the chemical includes dyspnea, respiratory depression, pulmonary edema, and respiratory paralysis. However, the effects of tetraethyl dithiopyrophosphate poisoning can take up to 12 hours to occur.
According to the Occupational Safety and Health Administration, the upper limit on exposure of tetraethyl dithiopyrophosphate to human skin is 0.2 milligrams per cubic meter.
Tetraethyl dithiopyrophosphate has toxic effects on spider mites, mealybugs, whiteflies, and aphids. However, the chemical is not phytotoxic, unlike tetraethyl pyrophosphate. However, it occasionally causes minor damage to plants, such as the slight puckering and cupping of leaves. During several tests in the late 1940s, it was found to be the most toxic of several chemicals to whiteflies on vegetables, two-spotted spider mites on roses, and mealybugs on numerous plants.
A mixture containing 5% tetraethyl dithiopyrophosphate at the concentration of 0.5 grams of phosphate per 1000 cubic feet was found in tests in the late 1940s to kill 100% of nonresistant two-spotted spider mites and 68% to 97% of resistant two-spotted spider mites. Tetraethyl dithiopyrophosphate aerosols killed 100% of the populations of a large number of insects, but only killed 98% of mealybugs in the same tests. 88% of nonresistant spider mites can be killed be two minutes of exposure to a mixture containing 5% of the chemical, 98% to 99% can be killed after five to ten minutes, and all can be killed after 15 minutes.
Tetraethyl dithiopyrophosphate is also toxic to wildlife, including fish and aquatic invertebrates. It is also assumed by the Environmental Protection Agency to be toxic to birds.
History
The first time that tetraethyl dithiopyrophosphate was registered to be used in the United States was in 1951. A Registration Standard for the chemical was issued by the Environmental Protection Agency in September 1988. Plans were made in 1999 by the Environmental Protection Agency to stop production of it by September 30, 2002 and to outlaw the use and distribution of products containing it by September 30, 2004.
