Formula TeBr4 Density 4.3 g/cm³ Melting point 388 °C Pubchem 82311 | Molar mass 447.22 g/mol Boiling point 421 °C Appearance yellow-orange crystals | |
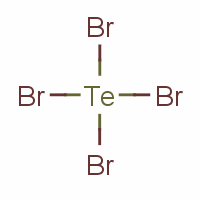 | ||
Tellurium tetrabromide (TeBr4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl4. It can be made by reacting bromine and tellurium. In the vapour TeBr4 dissociates:
It is a conductor when molten, dissociating into the ions TeBr3+ and Br− Solutions in benzene and toluene are non conducting and TeBr4 is present as the tetramer, Te4Br16. In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:
TeBr4 + 2CH3CN → (CH3CN)2TeBr3+ + Br−References
Tellurium tetrabromide Wikipedia(Text) CC BY-SA
