 | ||
The sulfur–iodine cycle (S–I cycle) is a three-step thermochemical cycle used to produce hydrogen.
Contents
The S–I cycle consists of three chemical reactions whose net reactant is water and whose net products are hydrogen and oxygen. All other chemicals are recycled. The S–I process requires an efficient source of heat.
Process description
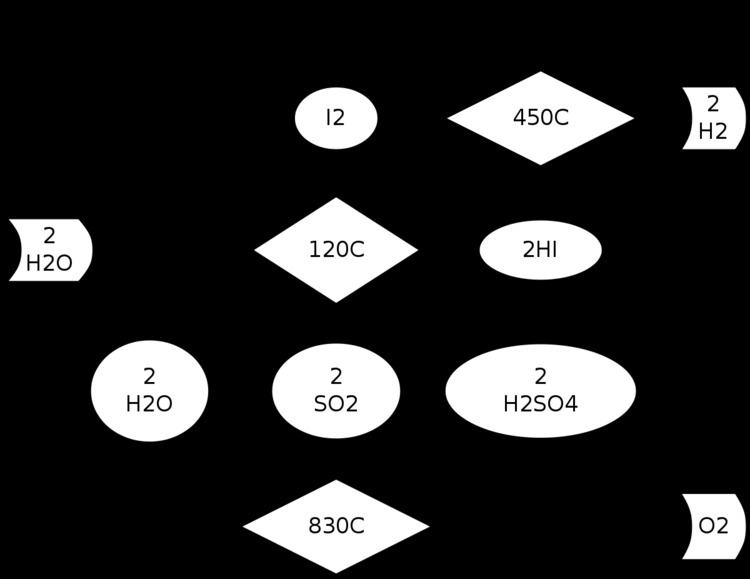
The three reactions that produce hydrogen are as follows:
- I2 + SO2 + 2 H2O → 2 HI + H2SO4 (120 °C); Bunsen reaction
- The HI is then separated by distillation or liquid/liquid gravitic separation.
- 2 H2SO4 → 2 SO2 + 2 H2O + O2 (830 °C)
- The water, SO2 and residual H2SO4 must be separated from the oxygen byproduct by condensation.
- 2 HI → I2 + H2 (450 °C)
- Iodine and any accompanying water or SO2 are separated by condensation, and the hydrogen product remains as a gas.
The sulfur and iodine compounds are recovered and reused, hence the consideration of the process as a cycle. This S–I process is a chemical heat engine. Heat enters the cycle in high-temperature endothermic chemical reactions 2 and 3, and heat exits the cycle in the low-temperature exothermic reaction 1. The difference between the heat entering and leaving the cycle exits the cycle in the form of the heat of combustion of the hydrogen produced.
Advantages and disadvantages
The characteristics of the S–I process can be described as follows:
Research
The S–I cycle was invented at General Atomics in the 1970s. The Japan Atomic Energy Agency (JAEA) has conducted successful experiments with the S–I cycle in the Helium cooled High Temperature Test Reactor, a reactor which reached first criticality in 1998, JAEA have the aspiration of using further nuclear high-temperature generation IV reactors to produce industrial scale quantities of hydrogen. (The Japanese refer to the cycle as the IS cycle.) Plans have been made to test larger-scale automated systems for hydrogen production. Under an International Nuclear Energy Research Initiative (INERI) agreement, the French CEA, General Atomics and Sandia National Laboratories are jointly developing the sulfur-iodine process. Additional research is taking place at the Idaho National Laboratory, in Canada, Korea and Italy.
Material challenge
The S–I cycle involves operations with corrosive chemicals at temperatures up to about 1,000 °C (1,830 °F). The selection of materials with sufficient corrosion resistance under the process conditions is of key importance to the economic viability of this process. The materials suggested include the following classes: refractory metals, reactive metals, superalloys, ceramics, polymers, and coatings. Some materials suggested include tantalum alloys, niobium alloys, noble metals, high-silicon steels, several nickel-based superalloys, mullite, silicon carbide (SiC), glass, silicon nitride (Si3N4), and others. Recent research on scaled prototyping suggests that new tantalum surface technologies may be a technically and economically feasible way to make larger scale installations.
Hydrogen economy
The sulfur-iodine cycle has been proposed as a way to supply hydrogen for a hydrogen-based economy. It does not require hydrocarbons like current methods of steam reforming but requires heat from combustion, nuclear reactions, or solar heat concentrators.
