 | ||
A speleothem ( /ˈspiːliːəθɛm/; Ancient Greek: "cave deposit"), commonly known as a cave formation, is a secondary mineral deposit formed in a cave. Speleothems typically form in limestone or dolostone solutional caves. The term "speleothem" as first introduced by Moore (1952), is derived from the Greek words spēlaion "cave" + théma "deposit". The definition of "speleothem" in most publications, specifically excludes secondary mineral deposits in mines, tunnels and on man-made structures. Hill and Forti more concisely defined "secondary minerals" which create speleothems in caves as;
Contents
- Origin and composition
- Types and categories
- Chemistry
- As climate proxies
- Absolute dating
- Calthemites secondary deposits not in caves
- References
A "secondary" mineral is one which is derived by a physicochemical reaction from a primary mineral in bedrock or detritus and/or deposited because of a unique set of conditions in a cave; i.e., the cave environment has influenced the mineral's deposition.
Origin and composition
More than 250 cave mineral deposits exist. The vast majority of speleothems are calcareous, composed of calcium carbonate in the form of calcite or aragonite, or calcium sulfate in the form of gypsum. Calcareous speleothems form via carbonate dissolution reactions. Rainwater in the soil zone reacts with soil CO2 to create weakly acidic water via the reaction:
H2O + CO2 → H2CO3As the lower pH water travels through the calcium carbonate bedrock from the surface to the cave ceiling, it dissolves the bedrock via the reaction:
CaCO3 + H2CO3 → Ca2+ + 2 HCO3−When the solution reaches a cave, degassing due to lower cave pCO2 drives precipitation of CaCO3:
Ca2+ + 2 HCO3− → CaCO3 + H2O + CO2Over time the accumulation of these precipitates form stalagmites, stalactites, and flowstones, which compose the major categories of speleothems.
Calthemites which occur on concrete structures, are created by completely different chemistry to speleothems.
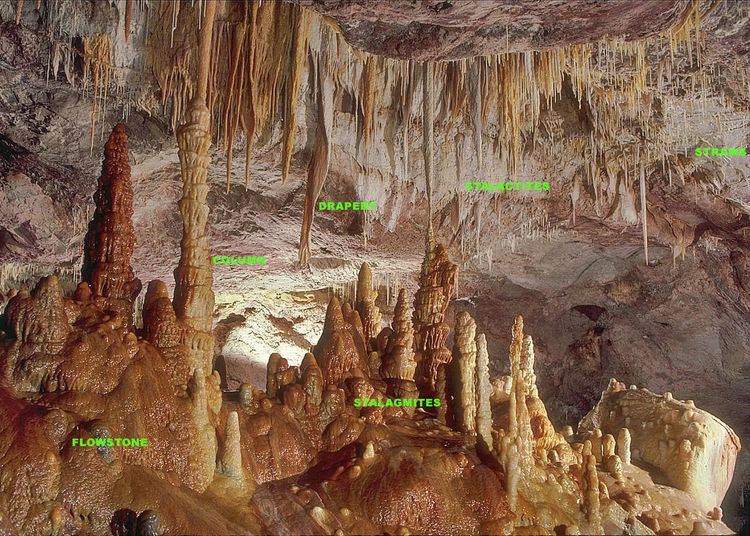
Types and categories
Speleothems take various forms, depending on whether the water drips, seeps, condenses, flows, or ponds. Many speleothems are named for their resemblance to man-made or natural objects. Types of speleothems include
Speleothems may also occur in lava tubes. Although sometimes similar in appearance to speleothems in caves formed by dissolution, these are formed by the cooling of residual lava within the lava tube.
Speleothems formed from salt, sulfur and other minerals are also known.
Speleothems made of pure calcium carbonate are a translucent white color, but often speleothems are colored by chemicals such as iron oxide, copper or manganese oxide, or may be brown because of mud and silt particulate inclusions.
Chemistry
Many factors impact the shape and color of speleothem formations including the rate and direction of water seepage, the amount of acid in the water, the temperature and humidity content of a cave, air currents, the above ground climate, the amount of annual rainfall and the density of the plant cover. Most cave chemistry revolves around calcium carbonate (CaCO3), the primary mineral in limestone and dolomite. It is a slightly soluble mineral whose solubility increases with the introduction of carbon dioxide (CO2). It is paradoxical in that its solubility decreases as the temperature increases, unlike the vast majority of dissolved solids. This decrease is due to interactions with the carbon dioxide, whose solubility is diminished by elevated temperatures; as the carbon dioxide is released, the calcium carbonate is precipitated.
Most other solution caves that are not composed of limestone or dolostone are composed of gypsum (calcium sulfate), the solubility of which is positively correlated with temperature.
As climate proxies
Samples can be taken from speleothems to be used like ice cores as a proxy record of past climate changes. A particular strength of speleothems in this regard is their unique ability to be accurately dated over much of the late Quaternary period using the uranium-thorium dating technique. Stalagmites are particularly useful for palaeoclimate applications because of their relatively simple geometry and because they contain several different climate records, such as oxygen and carbon isotopes and trace cations. These can provide clues to past precipitation, temperature, and vegetation changes over the last ~ 500,000 years.
Absolute dating
Another dating method using electron spin resonance (ESR) — also known as electron paramagnetic resonance (EPR) — is based on the measurement of electron-hole centers accumulated with time in the crystal lattice of CaCO3 exposed to natural radiations. In principle, in the more favorable cases, and assuming some simplifying hypotheses, the age of a speleothem could be derived from the total radiation dose cumulated by the sample and the annual dose rate to which it was exposed. Unfortunately, not all the samples are suited for ESR dating: indeed, the presence of cationic impurities such as Mn2+, Fe2+, or Fe3+, humic acids (organic matter), can mask the signal of interest, or interfere with it. Moreover, the radiation centers must be stable on geologic time, i.e., to have a very large lifetime, to make dating possible. Many other artifacts, such as, e.g., surface defects induced by the grinding of the sample can also preclude a correct dating. Only a few percents of the samples tested are in fact suitable for dating. This makes the technique often disappointing for the experimentalists. One of the main challenge of the technique is the correct identification of the radiation-induced centers and their great variety related to the nature and the variable concentration of the impurities present in the crystal lattice of the sample. ESR dating can be tricky and must be applied with discernment. It can never be used alone: "One date only is No date", or in other words, "multiple lines of evidence and multiple lines of reasoning are necessary in absolute dating". However, "good samples" might be found if all the selection criteria are met.
Calthemites: secondary deposits not in caves
Secondary deposits derived from concrete, lime, mortar or calcarious material as found on man-made structures outside the cave environment or in artificial caves (e.g. mines and tunnels), can mimic the shapes and forms of speleothems, but are classed as calthemites. The occurrence of calthemites is often associated with concrete degradation, but could also be linked to leaching of lime, mortar or other calcarious material (e.g. limestone and dolomite). Despite similar appearances, "calthemites" (created outside the cave environment) are not considered to be "speleothems" (created inside the cave environment), and vica versa, as per their definitions.
