Formula NaBO3·nH2O Appearance white powder | Molar mass 99.815 g/mol | |
 | ||
Sodium perborate
Sodium perborate (PBS) is a white, odorless, water-soluble chemical compound with the chemical formula Na2B2O4(OH)4. It is usually encountered as the tetrahydrate, but monohydrate, NaBO3·H2O and trihydrates are well known NaBO3·3H2O and The monohydrate and tetrahydrate are the commercially important forms. This salt is widely used in laundry detergents.
Contents
Preparation and reactions
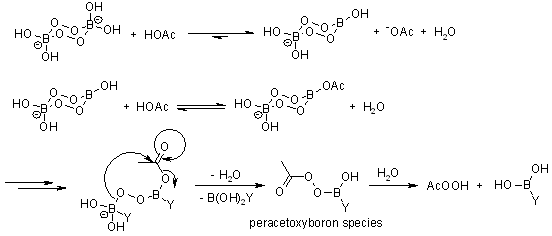
Sodium perborate is manufactured by reaction of disodium tetraborate pentahydrate, hydrogen peroxide, and sodium hydroxide. The monohydrate form dissolves better than the tetrahydrate and has higher heat stability; it is prepared by heating the tetrahydrate. Sodium perborate undergoes hydrolysis in contact with water, producing hydrogen peroxide and borate.
The salt is a reagent in organic synthesis. It converts thioethers into sulfoxides and sulfones for example.
Structure

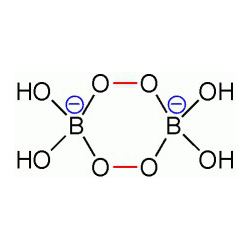
Unlike sodium percarbonate and perphosphate, the sodium perborate is not simply an adduct with hydrogen peroxide. Rather, it adopts a cyclic structure with a B2O4 core and four hydroxide groups attached to the two boron atoms. The ring has a chair-shaped 6-membered ring.
Uses
It serves as a source of active oxygen in many detergents, laundry detergents, cleaning products, and laundry bleaches. It is also present in some tooth bleaching formulas. It is used as a bleaching agent for internal bleaching of a non vital root treated tooth. The sodium perborate is placed inside the tooth and left in place for an extended period of time to allow it to diffuse into the tooth and bleach stains from the inside out. It has antiseptic properties and can act as a disinfectant. It is also used as a "disappearing" preservative in some brands of eye drops.
Sodium perborate is a less aggressive bleach than sodium hypochlorite, causing less degradation to dyes and textiles. Borates also have some non-oxidative bleaching properties.
Sodium perborate releases oxygen rapidly at temperatures over 60 °C. To make it active at lower temperatures (40–60 °C), it has to be mixed with a suitable activator, typically tetraacetylethylenediamine (TAED).
