Type Whole antibody Target IL-6 ATC code L04AC11 (WHO) | Trade names Sylvant | |
 | ||
Pregnancycategory US: C (Risk not ruled out) | ||
Siltuximab for mcd
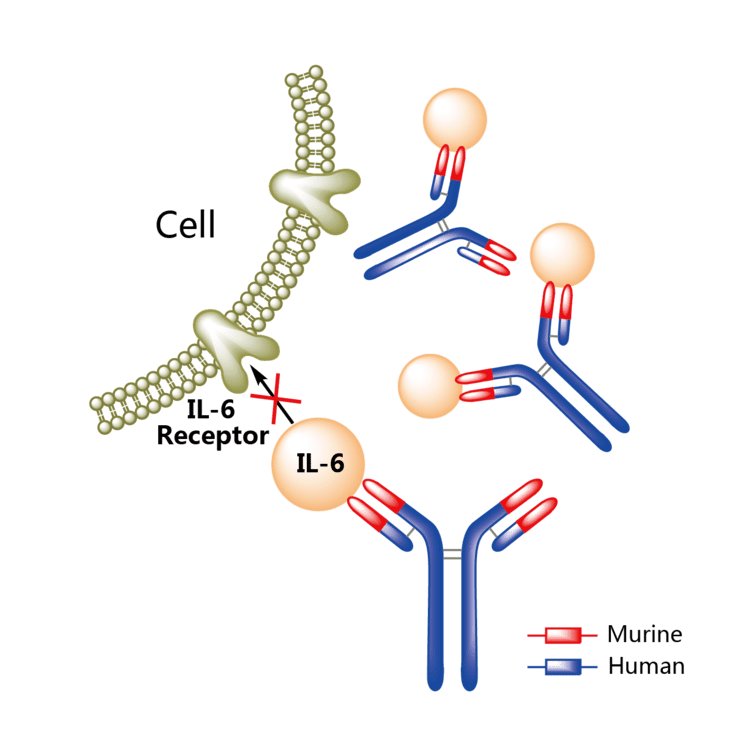
Siltuximab (INN, trade name Sylvant; also known as CNTO 328, anti-IL-6 chimeric monoclonal antibody or cCLB8) is a chimeric (made from human and mouse proteins) monoclonal antibody. It binds to interleukin-6. Siltuximab has been investigated for the treatment of neoplastic diseases: metastatic renal cell cancer, prostate cancer, and Castleman's disease, among other types of cancer.
Contents
It has undergone a phase I clinical trial in patients With B-cell non-Hodgkin's lymphoma, multiple myeloma, or Castleman's disease. There were encouraging results in a small trial for advanced ovarian cancer.
Encouraging results have been reported from a phase II trial for relapsed or refractory multiple myeloma.
On April 23, 2014, siltuximab was FDA approved under the brand name of Sylvant for the treatment of patients with multicentric Castleman’s disease (MCD) who do not have human immunodeficiency virus (HIV) or human herpesvirus-8 (HHV-8).
Medical uses
Used for the treatment of multicentric Castleman’s disease (MCD).
A randomized double-blind placebo controlled trial demonstrated that siltuximab treatment improved symptomatic multicenric Castleman’s disease (MCD) compared to placebo. Primary endpoints in the study included durable tumor and symptomatic response defined as complete or partial response with improvement or stabilization of disease related symptoms for at least 18 weeks.
A total of 79 patients were enrolled in the study (53 assigned to siltuximab, 26 assigned to placebo. This study included HIV-negative and human herpevisrus 8 (Kaposi's sarcoma-associated herpesvirus) seronegative patients aged 20-78 years old with symptomatic multicentric Castleman’s disease (fatigue, malaise, night sweats, peripheral sensory neuropathy, anorexia, pruritus, dyspnea, oedma, hyperhidrosis)
Side effects
Siltuximab may lower resistance to infections and should not be administered to patients with severe infections. Siltuximab should be discontinued in patients with severe infusion related reactions, anaphylaxis, severe allergic reactions or cytokine release syndromes. Live vaccines should not be administered to patients receiving siltuximab since IL-6 inhibition may interfere with normal immune response to new antigens.
Common The following has been shown to occur in treatment of Multicentric Castleman's disease with siltuximab during a clinical trial (>10% compared to placebo)
Long term exposure
Drug interactions
Siltuximab may increase CYP450 activity leading to increased metabolism of drugs that are CYP450 substrates. Co-administration of siltuximab and CYP450 substrates with narrow therapeutic index such as warfarin, ciclosporin or theophylline should be closely monitored.
Mechanism of action
Siltuximab is a chimeric monoclonal antibody that binds to interleukin-6 (IL-6), preventing binding to soluble and membrane bound interleukin-6 receptors. Siltuximab interferes with IL-6 mediated growth of B-lymphocytes and plasma cells, secretion of vascular endothelial growth factor (VEGF) and autoimmune phenomena.
