Formula C10H11ClZr Appearance White solid | Molar mass 257.87 g/mol | |
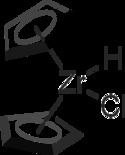 | ||
Schwartz's reagent is the common name for the chemical compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University. This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.
Contents
Preparation
The complex was first prepared by Wailes and Weigold. It can be purchased or readily prepared by reduction of zirconocene dichloride with lithium aluminium hydride:
(C5H5)2ZrCl2 + 1⁄4 LiAlH4 → (C5H5)2ZrHCl + 1⁄4 LiAlCl4In practice this reaction also affords (C5H5)2ZrH2, which is treated with methylene chloride to give the mixed hydride chloride. An alternative procedure that generated Schwartz's Reagent from dihydride has also been reported.
Uses in organic synthesis
Schwartz's reagent can be used for a number of reactions. It has been shown that it can be used to reduce amides to aldehydes. Reducing tertiary amides with Schwartz's reagent can reach efficient yields, but primary and secondary amides will show decreased yields. The use of Schwartz's reagent in this manner will not require any added heat and can be done quickly, and reduction of the alcohol form is not a problematic side reaction as it can be with other reducing agents. Schwartz's reagent will selectively reduce the amide over any readily reducible esters that may be present in the reaction mixture.
Vinylation of ketones in high yields is a possible use of Schwartz's reagent.
Schwartz's reagent is used in the synthesis of some macrolide antibiotics, (−)-motuporin, and antitumor agents.
Hydrozirconation
Schwartz's reagent reacts with alkenes and alkynes via the process called hydrozirconation, which results in the addition of the Zr–H bond across the C=C or C≡C bond.
The selectivity of the hydrozirconation of alkynes has been studied in detail. Generally, the addition of the Zr–H proceeds via the syn-addition. The rate of addition to unsaturated carbon-carbon bonds is terminal alkyne > terminal alkene ≈ internal alkyne > disubstituted alkene Acyl complexes can be generated by insertion of CO into the C–Zr bond resulting from hydrozirconation. Upon alkene insertion into the zirconium hydride bond, the resulting zirconium alkyl undergoes facile rearrangement to the terminal alkyl and therefore only terminal acyl compounds can be synthesized in this way. The rearrangement most likely proceeds via β-hydride elimination followed by reinsertion.
Computational studies indicate that hydrozirconation occurs from the interior portion.
