Formula Sc2S3 Melting point 1,775 °C Appearance yellow crystals | Molar mass 186.11 g/mol Density 2.91 g/cm³ | |
 | ||
Scandium(III) sulfide is a chemical compound of scandium and sulfur with the chemical formula Sc2S3. It is a yellow solid.
Contents
Structure
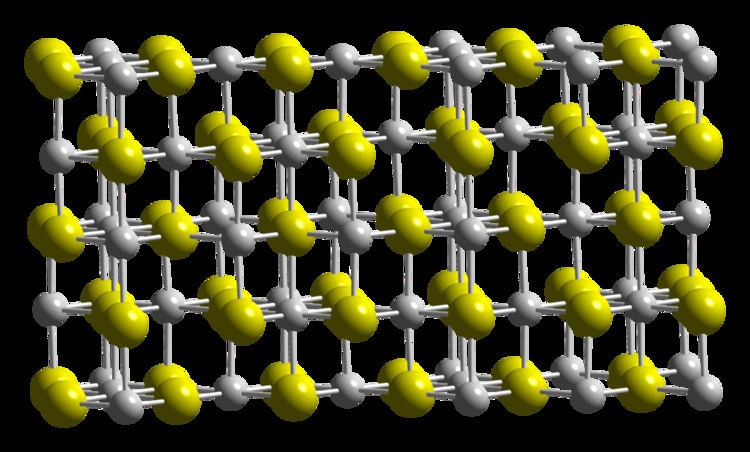
The crystal structure of Sc2S3 is closely related to that of sodium chloride, in that it is based on a cubic close packed array of anions. Whereas NaCl has all the octahedral interstices in the anion lattice occupied by cations, Sc2S3 has one third of them vacant. The vacancies are ordered, but in a very complicated pattern, leading to a large, orthorhombic unit cell belonging to the space group Fddd.
Synthesis
Metal sulfides are usually prepared by heating mixtures of the two elements, but in the case of scandium, this method yields scandium monosulfide, ScS. Sc2S3 can be prepared by heating scandium(III) oxide under flowing hydrogen sulfide in a graphite crucible to 1550 °C or above for 2–3 hours. The crude product is then purified by chemical vapor transport at 950 °C using iodine as the transport agent.
Sc2O3 + 3H2S → Sc2S3 + 3H2OReactivity
Above 1100 °C, Sc2S3 loses sulfur, forming nonstoichiometric compounds such as Sc1.37S2.
