Melting point 718 °C Molar mass 120.921 g/mol Density 2.8 g/cm³ | Formula RbCl Boiling point 1,390 °C | |
 | ||
Appearance white crystals; hygroscopic | ||
Rubidium chloride
Rubidium chloride is the chemical compound with the formula RbCl. This alkali metal halide is composed of rubidium and chlorine, and finds diverse uses ranging from electrochemistry to molecular biology.
Contents
- Rubidium chloride
- Structure
- Sodium chloride octahedral 66
- Caesium chloride cubic 88
- Sphalerite tetrahedral 44
- Synthesis
- Reactions
- Radioactivity
- Uses
- References
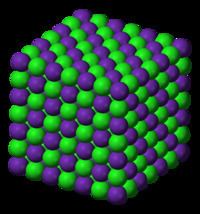
Structure
In its gas phase, RbCl is diatomic with a bond length estimated at 2.7868 Å. This distance increases to 3.285 Å for cubic RbCl, reflecting the higher coordination number of the ions in the solid phase.
Depending on conditions, solid RbCl exists in one of three arrangements or polymorphs as determined with holographic imaging:
Sodium chloride (octahedral 6:6)
The sodium chloride (NaCl) polymorph is most common. A cubic close-packed arrangement of chloride anions with rubidium cations filling the octahedral holes describes this polymorph. Both ions are six-coordinate in this arrangement. This polymorph's lattice energy is only 3.2 kJ/mol less than the following structure's.
Caesium chloride (cubic 8:8)
At high temperature and pressure, RbCl adopts the caesium chloride (CsCl) structure (NaCl and KCl undergo the same structural change at high pressures). Here, the chloride ions form a simple cubic arrangement with chloride anions occupying the vertices of a cube surrounding a central Rb+. This is RbCl's densest packing motif. Because a cube has eight vertices, both ions' coordination numbers equal eight. This is RbCl's highest possible coordination number. Therefore, according to the radius ratio rule, cations in this polymorph will reach their largest apparent radius because the anion-cation distances are greatest.
Sphalerite (tetrahedral 4:4)
The sphalerite polymorph of rubidium chloride is extremely rare, resulting in few structural studies. The lattice energy, however, for this formation is predicted to nearly 40.0 kJ/mol smaller than those of the preceding structures.
Synthesis
The most common preparation of pure rubidium chloride involves the reaction of its hydroxide with hydrochloric acid, followed by recrystallization:
RbOH(aq) + HCl(aq) → RbCl(aq) + H2O(l)Because RbCl is hygroscopic, it must be protected from atmospheric moisture, e.g. using a desiccator. RbCl is primarily used in laboratories. Therefore, numerous suppliers (see below) produce it in smaller quantities as needed. It is offered in a variety of forms for chemical and biomedical research.
Reactions
Rubidium chloride reacts with sulfuric acid to rubidium hydrogen sulfate.
Radioactivity
Every 18 mg of rubidium chloride is equivalent to approximately one banana equivalent dose due to the large fraction (27.8%) of naturally-occurring radioactive isotope rubidium-87.
