Formula C6AsNH6O6 | Molar mass 263.0365 g/mol | |
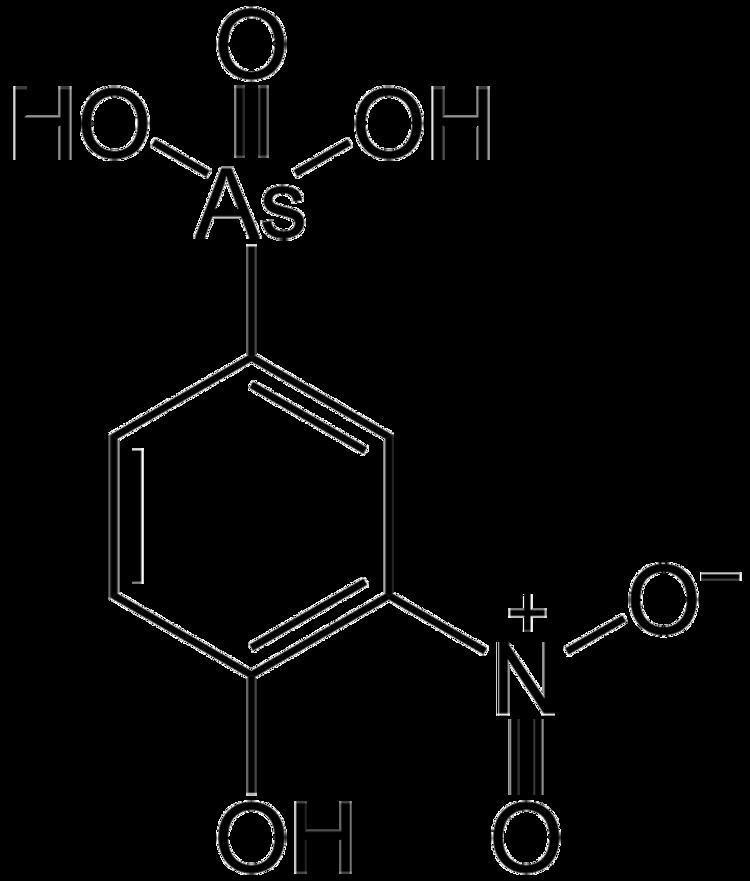 | ||
Roxarsone is an organoarsenic compound that is widely used in poultry production as a feed additive to increase weight gain and improve feed efficiency, and as a coccidiostat. The drug is also approved in the United States for use in pigs. Roxarsone is marketed as 3-Nitro by Zoetis, a former subsidiary of Pfizer now a publicly traded company. In 2006, approximately one million kilograms of roxarsone were produced in the U.S.
Contents
Roxarsone is one of four arsenical animal drugs approved by the U.S. Food and Drug Administration (FDA) for use in poultry and/or swine, along with nitarsone, arsanilic acid, and carbarsone. In September 2013, the FDA announced that Zoetis and Fleming Laboratories would voluntarily withdraw current roxarsone, arsanilic acid, and carbarsone approvals, leaving only nitarsone approvals in place. In 2015 FDA withdraw the approval of using nitarsone in animal feeds. The ban will came into effect at the end of 2015. Roxarsone is banned in the European Union.
Production and applications
Roxarsone is a derivative of phenylarsonic acid (C6H5As(O)(OH)2). It was first reported in a 1923 British patent that described the nitration and diazotization of arsanilic acid. When blended with calcite powder, it is used in poultry feed premixes and is usually available in 5%, 20% and 50% concentrations.
Controversy
Roxarsone has attracted attention as a source of arsenic contamination of poultry and other foods. In July 2011, Pfizer suspended sale of roxarsone in the U.S. in response to a study by the Food and Drug Administration (FDA); the FDA found that roxarsone use was associated with elevated levels of inorganic arsenic in chicken livers. An FDA press release stated that the findings raised "concerns of a very low but completely avoidable exposure to a carcinogen." The voluntary suspension does not preempt Pfizer from selling roxarsone in the future, as FDA has not withdrawn its approval to market the drug in the U.S. As of 2011, roxarsone was approved for use in 14 other countries as well.
A 2013 market basket study linked the use of roxarsone and other arsenical feed additives to increased levels of inorganic arsenic in chicken breast meat. Breast meat from conventionally produced chickens (in which arsenical use is permitted under U.S. law) had three times more inorganic arsenic than did breast meat from chickens produced according to USDA Organic standards. The USDA Organic program prohibits arsenical use in organic chicken production. The researchers also found that inorganic arsenic concentrations were almost three times higher in samples with detectable levels of roxarsone than in samples without detectable roxarsone.
