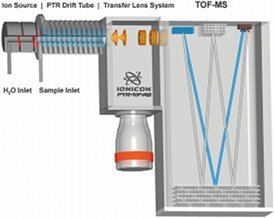
Proton-transfer-reaction mass spectrometry (PTR-MS) is an analytical chemistry technique that uses gas phase hydronium ions as ion source reagents. PTR-MS is used for online monitoring of volatile organic compounds (VOCs) in ambient air and was developed in 1995 by scientists at the Institut für Ionenphysik at the Leopold-Franzens University in Innsbruck, Austria. A PTR-MS instrument consists of an ion source that is directly connected to a drift tube (in contrast to SIFT-MS no mass filter is interconnected) and an analyzing system (quadrupole mass analyzer or time-of-flight mass spectrometer). Commercially available PTR-MS instruments have a response time of about 100 ms and reach a detection limit in the single digit pptv region. Established fields of application are environmental research, food and flavour science, biological research, medicine, etc.
Contents
Theory
With H3O+ as the primary ion the proton transfer process is (with R being the trace component)
Reaction (1) is only possible if energetically allowed, i.e. if the proton affinity of R is higher than the proton affinity of H2O (691 kJ/mol). As most components of ambient air possess a lower proton affinity than H2O (e.g. N2, O2, Ar, CO2, etc.) the H3O+ ions only reacts with VOC trace components and the air itself acts as a buffer gas. Moreover due to the low number of trace components one can assume that the total number of H3O+ ions remains nearly unchanged, which leads to the equation
In equation (2)
Technology
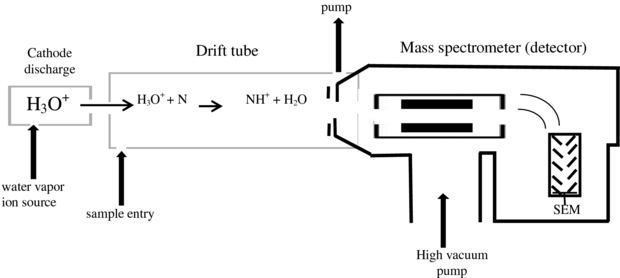
In commercial PTR-MS instruments water vapour is ionized in a hollow cathode discharge:
After the discharge a short drift tube is used to form very pure (>99.5%) H3O+ via ion-molecule reactions:
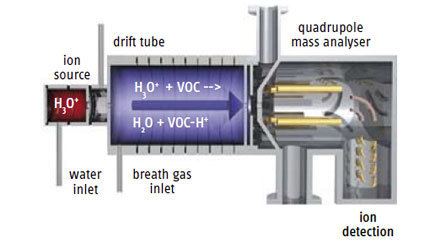

Due to the high purity of the primary ions a mass filter between the ion source and the reaction drift tube is not necessary and the H3O+ ions can be injected directly. The absence of this mass filter in turn greatly reduces losses of primary ions and leads eventually to an outstandingly low detection limit of the whole instrument. In the reaction drift tube a vacuum pump is continuously drawing through air containing the VOCs one wants to analyze. At the end of the drift tube the protonated molecules are mass analyzed (Quadrupole mass analyzer or Time-of-flight mass spectrometer) and detected.
Advantages of PTR-MS
Disadvantages of PTR-MS and countermeasures
Applications
The most common applications for the PTR-MS technique are (including some relevant publications):
Extensive reviews about PTR-MS and some of its applications were published in Mass Spectrometry Reviews by Joost de Gouw et al. (2007) and in Chemical Reviews by R.S. Blake et al. (2009). A special issue of the Journal of Breath Research dedicated to PTR-MS applications in medical research was published in 2009.
Food science
Fig. 2 shows a typical PTR-MS measurement performed in food and flavor research. The test person swallows a sip of a vanillin flavored drink and breathes via his nose into a heated inlet device coupled to a PTR-MS instrument. Due to the high time resolution and sensitivity of the instrument used here, the development of vanillin in the person's breath can be monitored in real-time (please note that isoprene is shown in this figure because it is a product of human metabolism and therefore acts as an indicator for the breath cycles). The data can be used for food design, i.e. for adjusting the intensity and duration of vanillin flavor tasted by the consumer.
Another example for the application of PTR-MS in food science was published in 2008 by C. Lindinger et al. in Analytical Chemistry. This publication found great response even in non-scientific media. Lindinger et al. developed a method to convert "dry" data from a PTR-MS instrument that measured headspace air from different coffee samples into expressions of flavour (e.g. "woody", "winey", "flowery", etc.) and showed that the obtained flavor profiles matched nicely to the ones created by a panel of European coffee tasting experts.
Air quality analysis
In Fig. 3 a mass spectrum of air inside a laboratory (obtained with a time-of-flight (TOF) based PTR-MS instrument), is shown. The peaks on masses 19, 37 and 55 m/z (and their isotopes) represent the reagent ions (H3O+) and their clusters. On 30 and 32 m/z NO+ and O2+, which are both impurities originating from the ion source, appear. All other peaks correspond to compounds present in typical laboratory air (e.g. high intensity of protonated acetone on 59 m/z). If one takes into account that virtually all peaks visible in Fig. 3 are in fact double, triple or multiple peaks (isobaric compounds) it becomes obivious that for PTR-MS instruments selectivity is at least as important as sensitivity, especially when complex samples / compositions are analyzed. Methods to handle this issue have been suggestested in literature as:
