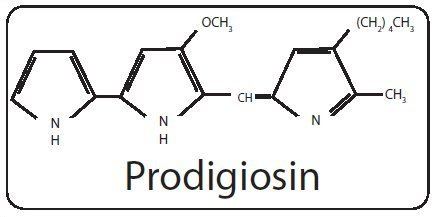
Molar mass 323.432 g/mol | Formula C20H25N3O | |
 | ||
Prodigiosin is the red pigment produced by many strains of the bacterium Serratia marcescens, other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis It is in a family of compounds termed "prodiginines", which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria (e.g. Streptomyces coelicolor). The name "prodigiosin" is derived from "prodigious" (i.e. something marvelous).
Contents

Secondary metabolite

Prodigiosin is a secondary metabolite of Serratia marcescens. Because it is easy to detect, it has been used as a model system to study secondary metabolism. Prodigiosin production has long been known to be enhanced by phosphate limitation. In low phosphate conditions, pigmented strains have been shown to grow to a higher density than unpigmented strains.
Religious function

The ability of pigmented strains of Serratia marcescens to grow on bread has led to a possible explanation of Medieval transubstantiation miracles, in which Eucharistic bread is converted into the Body of Christ. Such miracles led to Pope Urban IV instituting the Feast of Corpus Christi in 1264. This followed celebration of a Mass at Bolsena in 1263, led by a Bohemian priest who had doubts concerning transubstantiation. During the Mass, the eucharist appeared to bleed and each time the priest wiped away the blood, more would appear. This event is celebrated in a fresco in the Pontifical Palace in the Vatican City, painted by Raphael.
Biological activity

Prodigiosin has recently received renewed attention for its wide range of biological activities, including activities as antimalarial, antifungal, immunosuppressant, and antibiotic agents. It is perhaps best known for its capacity to trigger apoptosis of malignant cancer cells. The exact mechanism of this inhibition is highly complex and not entirely elucidated, but could involve multiple processes, including phosphatase inhibition, copper mediated cleavage of double stranded DNA, or disrupting the pH gradient through transmembrane transport of H+ and Cl- ions. As a result, Prodigiosin is a highly promising drug lead, and is currently in preclinical phase study for pancreatic cancer treatment. Prodigiosin has recently been found to have excellent activity against stationary phase Borrelia burgdorferi, the causative agent of Lyme Disease.
Biosynthesis
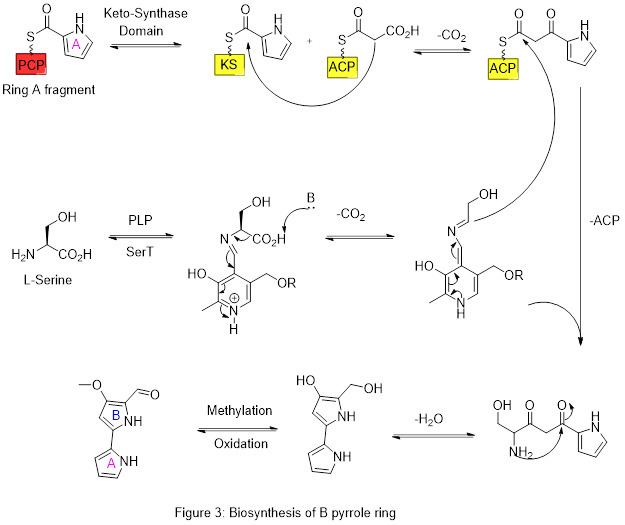
The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-Serine, L-methionine, pyruvate, and 2-octenal.
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-Serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A-B ring structure.
Ring C is formed from the TPP mediated decarboxylative addition of pyruvate to 2-octenal, followed by reaction with PLP to generate an amine for intramolecular condensation. Oxidation of the resulting ring yields the final pyrrole ring C.
Finally, these two pieces are stitched together in a dehydration reaction driven forward by the establishment of a conjugated system across all three rings. This completes the convergent synthesis of the natural product.
