Related compounds Molar mass 94.2 g/mol Appearance Pale yellow solid | Formula K2O Density 2.35 g/cm³ | |
 | ||
Ionic bonding of lithium fluoride potassium oxide chemistry for all fuseschool
Potassium oxide (K2O) is an ionic compound of potassium and oxygen. This pale yellow solid, the simplest oxide of potassium, is a rarely encountered, highly reactive compound. Some materials of commerce, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to the chemical compoud mixture K2O.
Contents
- Ionic bonding of lithium fluoride potassium oxide chemistry for all fuseschool
- Production
- Properties and reactions
- Term use in industry
- References
Production
Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium oxide, K2O. Treatment of the peroxide with potassium produces the oxide:
K2O2 + 2 K → 2 K2OAlternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium:
2 KNO3 + 10 K → 6 K2O + N2Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen.
2 K2O2Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hydrogen as a byproduct.
Properties and reactions
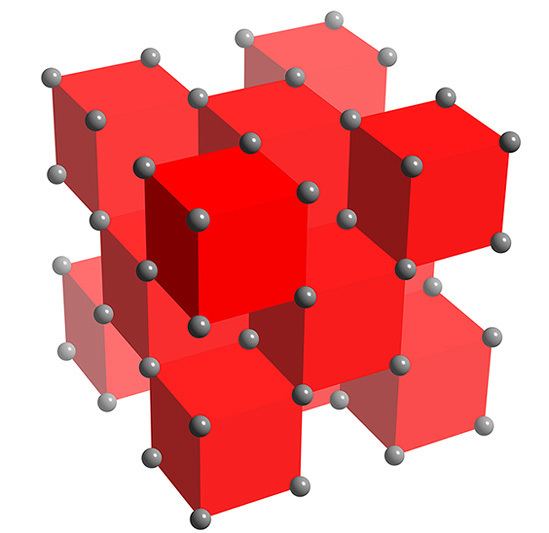
K2O crystallises in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in CaF2, with potassium ions coordinated to 4 oxide ions and oxide ions coordinated to 8 potassium. K2O is a basic oxide and reacts with water violently to produce the caustic potassium hydroxide. It is deliquescent and will absorb water from the atmosphere, initiating this vigorous reaction.
Term use in industry
The chemical formula K2O (or simply 'K') is used in several industrial contexts: the N-P-K numbers for fertilizers, in cement formulas, and in glassmaking formulas. Potassium oxide is often not used directly in these products, but the amount of potassium is reported in terms of the K2O equivalent for whichever type of potash was used, such as potassium carbonate. For example, potassium oxide is about 83% potassium by weight, while potassium chloride is only 52%. Potassium chloride provides less potassium than an equal amount of potassium oxide. Thus, if a fertilizer is 30% potassium chloride by weight, its standard potassium rating, based on potassium oxide, would be only 18.8%.
