Formula K2MnO4 Melting point 190 °C Appearance dark green crystals | Molar mass 197.132 g/mol Density 2.78 g/cm³ | |
 | ||
Related compounds | ||
Oxidation of iron ii to iron iii using potassium manganate vii
Potassium manganate is the inorganic compound with the formula K2MnO4. This green-colored salt is an intermediate in the industrial synthesis of potassium permanganate (KMnO4), a common chemical. Occasionally, potassium manganate and potassium permanganate are confused, but they are different compounds with distinctly different properties.
Contents
Structure
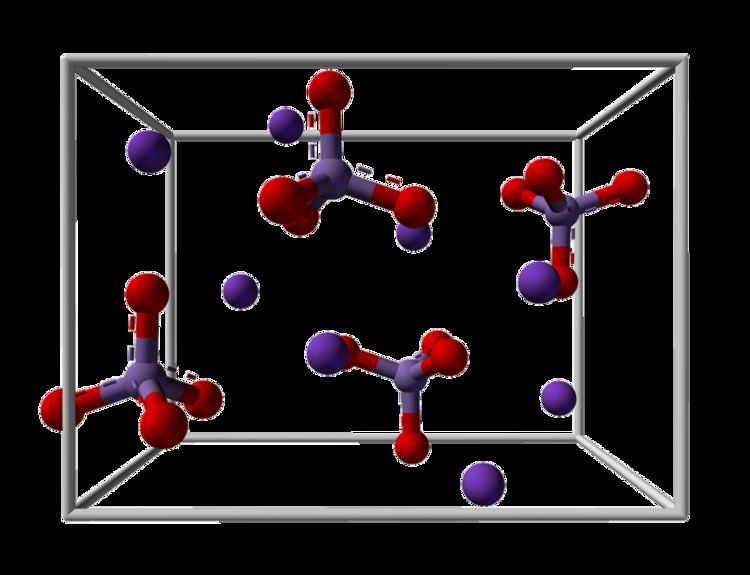
K2MnO4 is a salt, consisting of K+ cations and MnO42− anions. X-ray crystallography shows that the anion is tetrahedral, with Mn-O distances of 1.66 Å, ca. 0.03 Å longer than the Mn-O distances in KMnO4. It is isostructural with potassium sulfate.
Synthesis
The industrial route entails treatment of MnO2 with air:
2 MnO2 + 4 KOH + O2 → 2 K2MnO4 + 2 H2OThe transformation gives a green-colored melt. One can test an unknown substance for the presence of manganese by heating the sample in strong KOH in air. The production of a green coloration indicates the presence of Mn. This green color results from an intense absorption at 610 nm.
In the laboratory, K2MnO4 can be synthesized by heating a solution of KMnO4 in concentrated KOH solution followed by cooling to give green crystals:
4 KMnO4 + 4 KOH → 4 K2MnO4 + O2 + 2 H2OThis reaction illustrates the relatively rare role of hydroxide as a reducing agent. Solutions of K2MnO4 are generated by allowing a solution of KMnO4 in 5–10 M KOH to stir for a day at room temperature followed by removal of MnO2, which is insoluble. The concentration of K2MnO4 in such solutions can be checked by measuring their absorbance at 610 nm.
The one-electron reduction of permanganate to manganate can also be effected using iodide as the reducing agent:
2 KMnO4 + 2 KI → 2 K2MnO4 + I2The conversion is signaled by the color change from purple, characteristic of permanganate, to the green color of manganate. This reaction also shows that manganate(VII) can serve as an electron acceptor in addition to its usual role as an oxygen-transfer reagent. Barium manganate, BaMnO4, is generated by the reduction of KMnO4 with iodide in the presence of barium chloride. Just like BaSO4, BaMnO4 exhibits low solubility in virtually all solvents.
An easy method for preparing potassium manganate in the laboratory involves heating crystals or powder of pure potassium permanganate. Potassium permanganate will decompose into potassium manganate, manganese dioxide and oxygen gas:
2KMnO4 → K2MnO4 + MnO2 + O2This reaction is a laboratory method to prepare oxygen, but produces samples of potassium manganate contaminated with MnO2.
Reactions
Manganate salts readily disproportionate to permanganate ion and manganese dioxide:
3 K2MnO4 + 2 H2O → 2 KMnO4 + MnO2 + 4 KOHThe colorful nature of the disproportionation has led the manganate/manganate(VII) pair to be referred to as a chemical chameleon. This disproportionation reaction, which becomes rapid when [OH−
] < 1M, follows bimolecular kinetics.[1]
