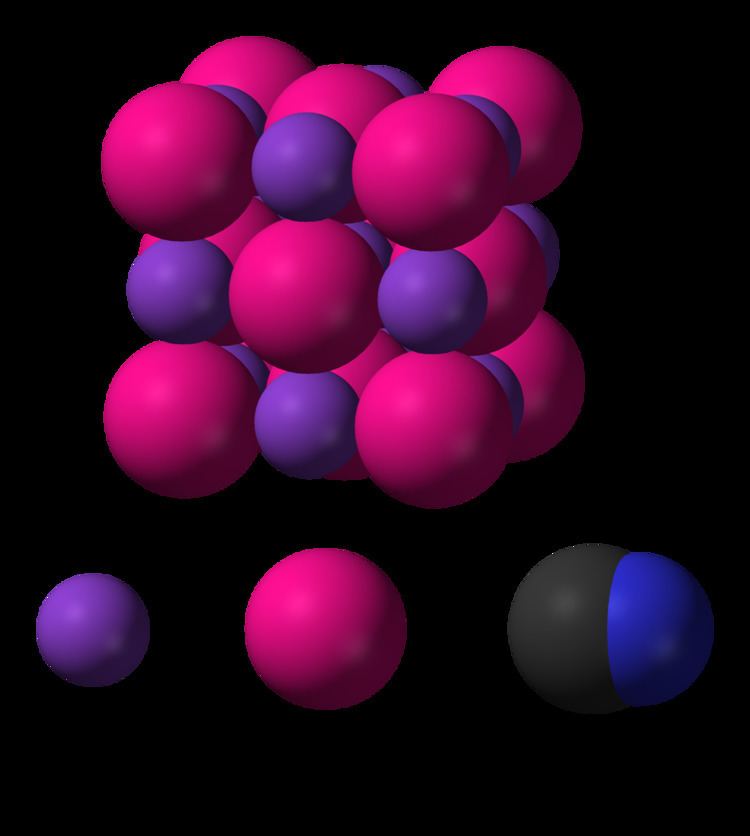
Related compounds IUPAC ID Potassium cyanide Density 1.52 g/cm³ Boiling point 1,625 °C | Formula KCN Molar mass 65.12 g/mol Melting point 634.5 °C Soluble in Water | |
 | ||
Appearance White crystalline solid
deliquescent | ||
Potassium cyanide
Potassium cyanide is a compound with the formula KCN. This colorless crystalline salt, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and buffing.
Contents
- Potassium cyanide
- Production
- Historical production
- Structure
- Applications
- Potassium gold cyanide
- Toxicity
- References
Potassium cyanide is highly toxic. The moist solid emits small amounts of hydrogen cyanide due to hydrolysis, which smells like bitter almonds. Not everyone, however, can smell this; the ability to do so is a genetic trait.

The taste of potassium cyanide has been described as acrid with a burning sensation.
Production

KCN is produced by treating hydrogen cyanide with aqueous solution of potassium hydroxide, followed by evaporation of the solution in a vacuum:
HCN + KOH → KCN + H2Oor by treating formamide with potassium hydroxide:
HCONH2 + KOH → KCN + 2H2OAbout 50,000 tons of potassium cyanide are produced yearly.
Historical production
Prior to 1900 AD, before the invention of the Castner process, potassium cyanide was the most important source of alkali metal cyanides. In this historical process, potassium cyanide was produced by decomposing potassium ferrocyanide:
K4[Fe(CN)6] → 4 KCN + FeC2 + N2
Structure

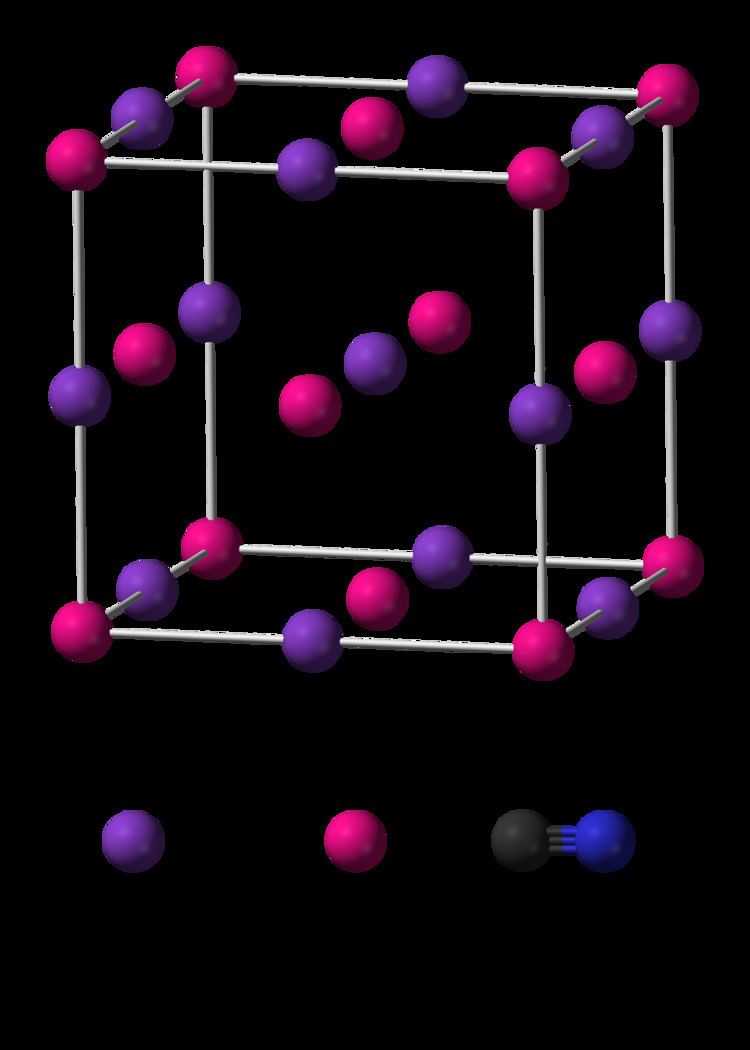
In aqueous solution, KCN is dissociated into hydrated potassium (K+) ions and cyanide (CN−) ions. The common form of solid KCN, stable at ambient pressure and temperature, has the same cubic crystal structure as sodium chloride, with each potassium ion surrounded by six cyanide ions, and vice versa. Despite the cyanide ions being diatomic, and thus less symmetric than chloride, they rotate so rapidly, their time-averaged shape is spherical. At low temperature and high pressure, this free rotation is hindered, resulting in a less symmetric crystal structure with the cyanide ions arranged in sheets.
Applications
KCN and sodium cyanide (NaCN) are widely used in organic synthesis for the preparation of nitriles and carboxylic acids, particularly in the von Richter reaction. It also finds use for the synthesis of hydantoins, which can be useful synthetic intermediates, when reacted with a carbonyl compound such as an aldehyde or ketone in the presence of ammonium carbonate.
KCN is used as a photographic fixer in the wet plate collodion process. The KCN dissolves silver where it has not been made insoluble by the developer. This reveals and stabilizes the image, making it no longer sensitive to light. Modern wet plate photographers may prefer less toxic fixers, often opting for the less toxic Sodium thiosulphate, but KCN is still used.
Potassium gold cyanide
In gold mining, KCN forms the water-soluble salt potassium gold cyanide (or gold potassium cyanide) and potassium hydroxide from gold metal in the presence of oxygen (usually from the surrounding air) and water:
4 Au + 8 KCN + O2 + 2 H2O → 4 K[Au(CN)2] + 4 KOHA similar process uses NaCN to produce sodium gold cyanide (NaAu(CN2)).
Toxicity
Potassium cyanide is a potent inhibitor of cellular respiration, acting on mitochondrial cytochrome c oxidase, hence blocking oxidative phosphorylation. This prevents the body from oxidizing food to produce useful energy. Lactic acidosis then occurs as a consequence of anaerobic metabolism. Initially, acute cyanide poisoning causes a red or ruddy complexion in the victim because the tissues are not able to use the oxygen in the blood. The effects of potassium and sodium cyanide are identical, and symptoms of poisoning typically occur within a few minutes of ingesting the substance: the person loses consciousness, and brain death eventually follows. During this period the victim may suffer convulsions. Death is caused by cerebral hypoxia.
The lethal dose for potassium cyanide is 200–300 mg. Its toxicity when ingested depends on the acidity of the stomach, because it must react with an acid to become hydrogen cyanide, the deadly form of cyanide. Grigori Rasputin may have survived a potassium cyanide poisoning because his stomach acidity was unusually low.
A number of prominent persons were killed or committed suicide using potassium cyanide, including members of the Young Bosnia and famous personalities in the Third Reich, such as Erwin Rommel, Hitler's longtime companion Eva Braun, Joseph Goebbels, Heinrich Himmler, and Hermann Göring. World War II era British agents (using purpose-made suicide pills), computer scientist Alan Turing, polymer chemist Wallace Carothers, 19th-Century German chemist Viktor Meyer, and various religious cult suicides such as by the Peoples Temple. Danish writer Gustav Wied and members of the LTTE involved in the assassination of Indian prime minister Rajiv Gandhi. Jason Altom, who was a promising graduate student in the lab of Nobel-Prizewinning chemist EJ Corey at Harvard.
It is used by professional entomologists as a killing agent in collecting jars, as insects succumb within seconds to the HCN fumes it emits, thereby minimizing damage to even highly fragile specimens.
KCN can be detoxified most efficiently with hydrogen peroxide or with a solution of sodium hypochlorite. Such solutions should be kept basic whenever possible so as to eliminate the possibility of generation of hydrogen cyanide:
KCN + H2O2 → KOCN + H2O