 | ||
The polyiodides are a class of polyhalogen anions composed of entirely iodine atoms. The most common and simplest member is the triiodide ion, I−
3. Other known, larger polyiodides include [I4]2−, [I5]−, [I7]−, [I8]2−, [I9]−, [I10]2−, [I10]4−, [I11]3−, [I12]2−, [I13]3−, [I16]2−, [I22]4−, [I26]3−, [I26]4−, [I28]4− and [I29]3−.
Contents
Preparation
The polyiodides can be made by addition of stoichiometric amounts of I2 to solutions containing I− and I−
3, with the presence of large counter-cations to stabilize them. For example, KI3·H2O can be crystallized from a saturated solution of KI when a stoichiometric amount of I2 is added and cooled.
Structure
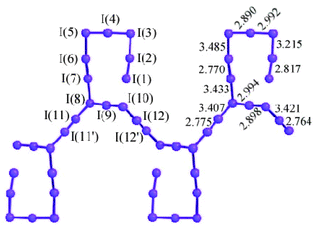
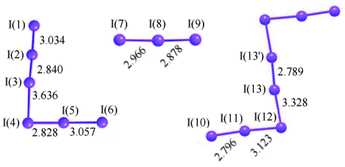
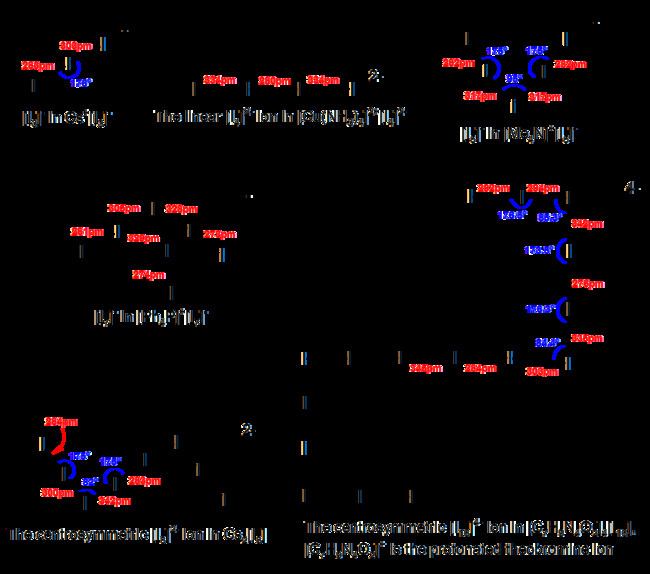
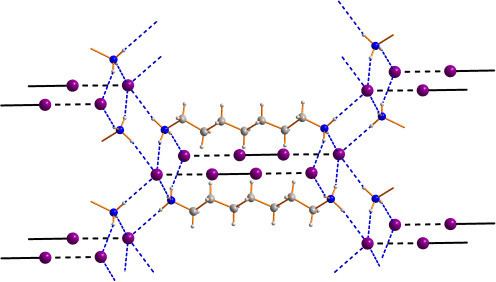
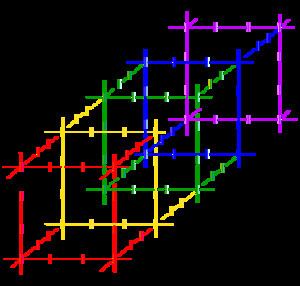
Polyiodides are characterized by their highly complex and variable structures, and can be considered as associations of I2, I−, and I−
3 units. Discrete polyiodides are usually linear, reflecting the origin of the ion. The more complex two- or three-dimensional network structures of chains and cages are formed as the ions interact with each other, with their shapes depending on their associated cations quite strongly. The table below lists the polyiodide salts which have been structurally characterized, along with their counter-cation.