Type ? Target CD79b CAS Number 1313206-42-6 | ATC code none IUPHAR/BPS 8404 | |
 | ||
Two doses of polatuzumab vedotin in patients with relapsed refractory follicular lymphoma
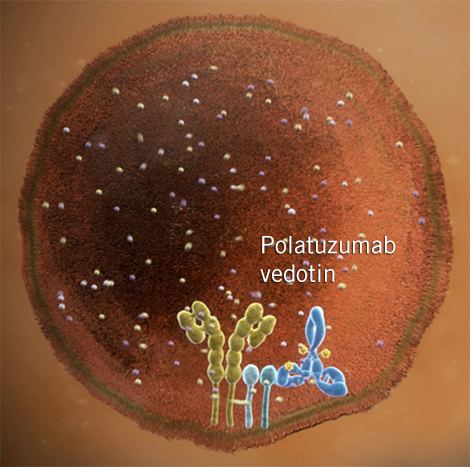
Polatuzumab vedotin (DCDS4501A or RG7596) is an antibody-drug conjugate or ADC designed for the treatment of cancer.
Contents
- Two doses of polatuzumab vedotin in patients with relapsed refractory follicular lymphoma
- Mechanism of Action
- Clinical trials
- References
The investigational drug, being developed by Genentech/Roche, contains a humanized monoclonal antibody (mAb) targeting CD79b (B-cell antigen receptor complex-associated protein beta chain) conjugated to the synthetic dolastatin 10 analog microtubule-disrupting monomethyl auristatin E (MMAE) through engineered cysteines (THIOMABs) via a protease-cleavable peptide linker (valine–citrulline; Maleimidocaproylvaline-citrulline-p-aminobenzoyloxycarbonyl or MC-VC-PABC). The advantage of the citrulline-valine (VC) linker being used that it is highly stable in plasma.
Mechanism of Action
Upon administration, polatuzumab vedotin selectively binds to CD79b, a protein which is abundantly expressed on the surface of B-cells. Following internalization and proteolytic cleavage, MMAE binds to tubulin and inhibits its polymerization, resulting in G2/M phase arrest and tumor cell apoptosis.
Clinical trials
Polatuzumab vedotin is being investigated in Phase I and Phase II clinical trials. The Phase I trial investigates the drug in B-cell non-Hodgkin’s lymphoma while the Phase II trials investigates the trial drug for the treatment of patients with relapsed or refractory follicular or diffuse large B-Cell Lymphoma.
