 | ||
Photoelectrochemical reduction of CO2 is a chemical process whereby carbon dioxide is reduced to carbon monoxide or hydrocarbons by the energy of incident light. This process needs to be catalyzed either homogeneously or heterogeneously in order to proceed, and current research is aimed at developing these catalysts, most of which are semiconducting materials. Semiconducting catalysts provide favourable electron transfer kinetics.
Contents
- BackgroundIntroduction
- Thermodynamic challenges
- Kinetic challenges
- Is it feasible to photoelectrochemically reduce CO2 on semiconductor surface
- Solvent effect
- Aqueous media
- Non aqueous media
- References
Motivation for research in this area is strong due to the current attention to atmospheric carbon dioxide as the reduction of carbon dioxide would be one route for removal and sequestration. Furthermore, the reduced species may prove to be a valuable feedstock for other processes. If the incident light utilized is solar in nature then this process also potentially represents energy routes which combine renewable energy with CO2 reduction.
Background/Introduction
Semiconductor material has a band gap and generates a pair of electron and hole per absorbed photon if the energy of photon is higher than band gap of semiconductor. This property of semiconductor materials has been successfully used to convert solar energy into electrical energy by photovoltaic devices. So, semiconductor electrodes can be used for CO2 photoelectrochemical reduction.
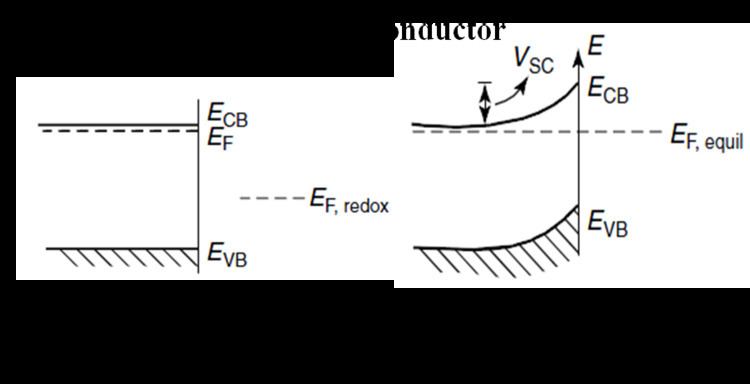
When a semiconductor comes into contact with a liquid (redox species), to maintain electrostatic equilibrium, there will be a charge transfer between the semiconductor and liquid phase if formal redox potential of redox species lies inside semiconductor band gap. At thermodynamic equilibrium, the Fermi level of semiconductor and the formal redox potential of redox species are aligned at the interface between semiconductor and redox species. This introduces a downward band bending in a n-type semiconductor for n-type semiconductor/liquid junction (Figure 1) and upward band bending in a p-type semiconductor for p-type semiconductor/liquid junction (Figure 2). This characteristic of semiconductor/liquid junction is similar to a rectifying semiconductor/metal junction or Schottky junction. Ideally to get a good rectifying characteristics at the semiconductor/liquid interface, the formal redox potential must be close to the valence band of the semiconductor for a n-type semiconductor and close to the conduction band of the semiconductor for a p-type semiconductor. The semiconductor/liquid junction has one advantage over rectifying semiconductor/metal junction in that the light is able to travel through to semiconductor surface without much reflection; whereas most of the light is reflected back from metal surface at semiconductor/metal junction. Therefore, semiconductor/liquid junction can also be used a photovoltaic devices similar to solid state p–n junction devices. Both n-type and p-type semiconductor/liquid junction can be used as photovoltaic devices to convert solar energy into electrical energy and are called photoelectrochemical cell. In addition, a semiconductor/liquid junction could also be used to directly convert solar energy into chemical energy by virtue of photoelectrolysis at the semiconductor/liquid junction.
The catalytic conversion of CO2 to liquid fuels is a critical goal that would positively impact the global carbon balance by recycling CO2 into usable fuels. The challenges presented here are great, but the potential rewards are enormous. CO2 is extremely stable molecule generally produced by fossil fuel combustion and respiration. Returning CO2 to a useful state by activation/reduction is a scientifically challenging problem, requiring appropriate catalysts and energy input. This poses several fundamental challenges in chemical catalysis, electrochemistry, photochemistry, and semiconductor physics and engineering.
Thermodynamic challenges
Thermodynamic potentials for the reduction of CO2 to various products is given in the following table versus NHE at pH = 7. Single electron reduction of CO2 to CO2●− radical occurs at E° = −1.90 V versus NHE at pH = 7 in an aqueous solution at 25 °C under 1 atm gas pressure. The reason behind the high negative thermodynamically unfavorable single electron reduction potential of CO2 is the large reorganization energy between the linear molecule and bent radical anion. Proton-coupled multi-electron steps for CO2 reductions are generally more favorable than single electron reductions, as thermodynamically more stable molecules are produced.
Kinetic challenges
Thermodynamically, proton coupled multiple-electron reduction of CO2 is easier than single electron reduction. But to manage multiple proton coupled multiple-electron processes is a huge challenge kinetically. This leads to a high overpotential for electrochemical heterogeneous reduction of CO2 to hydrocarbons and alcohols. Even further heterogeneous reduction of singly reduced CO2●− radical anion is difficult because of repulsive interaction between negatively biased electrode and negatively charged anion.
Is it feasible to photoelectrochemically reduce CO2 on semiconductor surface?
Figure 1(b) shows that in case of a p-type semiconductor/liquid junction photo generated electrons are available at the semiconductor/liquid interface under illumination. The reduction of redox species happens at less negative potential on illuminated p-type semiconductor compared to metal electrode due to the band bending at semiconductor/liquid interface. Figure 3 shows that thermodynamically, some of the proton-coupled multi-electron CO2 reductions are within semiconductors band gap. This makes it feasible to photo-reduce CO2 on p-type semiconductors. Various p-type semiconductors have been successfully employed for CO2 photo reduction including p-GaP, p-CdTe, p-Si, p-GaAs, p-InP, and p-SiC. Kinetically, however, these reactions are extremely slow on given semiconductor surfaces; this leads to significant overpotential for CO2 reduction on these semiconductor surfaces. Apart from high overpotential; these systems have a few advantages including sustainability (nothing is consumed in this system apart from light energy), direct conversion of solar energy to chemical energy, utilization of renewable energy resource for energy intensive process, stability of the process (semiconductors are really stable under illumination) etc. A different approach for photo-reduction of CO2 involves molecular catalysts, photosensitizers and sacrificial electron donors. In this process sacrificial electron donors are consumed during the process and photosensitizers degrade under long exposure to illumination.
Solvent effect
The photo-reduction of CO2 on p-type semiconductor photo-electrodes has been achieved in both aqueous and non-aqueous media. Main difference between aqueous and non-aqueous media is the solubility of CO2. The solubility of CO2 in aqueous media at 1 atm. of CO2 is around ≈ 35 mM; whereas solubility of CO2 in methanol is around 210 mM and in acetonitrile is around 210 mM.
Aqueous media
Halmann had first shown CO2 photoreduction to formic acid on p-GaP as photocathode in aqueous media in 1978. Apart from several other reports of CO2 photoreduction on p-GaP, there are other p-type semiconductors like p-GaAs, p-InP, p-CdTe, and p+/p-Si have been successfully used for photoreduction of CO2. The lowest potential for CO2 photoreduction was observed on p-GaP. This may be due to high photovoltage excepted from higher band gap p-GaP (2.2 eV) photocathode. Apart from formic acid, other products observed for CO2 photoreduction are formaldehyde, methanol and carbon monoxide. On p-GaP, p-GaAs and p+/p-Si photocathode, the main product is formic acid with small amount of formaldehyde and methanol. However, for p-InP and p-CdTe photocathode, both carbon monoxide and formic acid are observed in similar quantities. Mechanism proposed by Hori based on CO2 reduction on metal electrodes predicts formation of both formic acid (in case of no adsorption of singly reduced CO2●− radical anion to the surface) and carbon monoxide (in case of adsorption of singly reduced CO2●− radical anion to the surface) in aqueous media. This same mechanism can be evoked to explain the formation of mainly formic acid on p-GaP, p-GaAs and p+/p-Si photocathode owing to no adsorption of singly reduced CO2●− radical anion to the surface. In case of p-InP and p-CdTe photocathode, partial adsorption of CO2●− radical anion leads to formation of both carbon monoxide and formic acid. Low catalytic current density for CO2 photoreduction and competitive hydrogen generation are two major drawbacks of this system.
Non-aqueous media
Maximum catalytic current density for CO2 reduction that can be achieved in aqueous media is only 10 mA cm−2 based solubility of CO2 and diffusion limitations. The integrated maximum photocurrent under Air Mass 1.5 illumination, in the conventional Shockley-Quiesser limit for solar energy conversion for p-Si (1.12 eV), p-InP (1.3 eV), p-GaAs (1.4 eV), and p-GaP (2.3 eV) are 44.0 mA cm−2, 37.0 mA cm−2, 32.5 mA cm−2 and 9.0 mA cm−2, respectively. Therefore, non-aqueous media such as DMF, acetonitrile, methanol are explored as solvent for CO2 electrochemical reduction. In addition, Methanol has been industrially used as a physical absorber of CO2 in the Rectisol method. Similarly to aqueous media system, p-Si, p-InP, p-GaAs, p-GaP and p-CdTe are explored for CO2 photoelectrochemical reduction. Among these, p-GaP has lowest overpotential, whereas, p-CdTe has moderate overpotential but high catalytic current density in DMF with 5% water mixture system. Main product of CO2 reduction in non-aqueous media is carbon monoxide. Competitive hydrogen generation is minimized in non-aqueous media. Proposed mechanism for CO2 reduction to CO in non-aqueous media involves single electron reduction of CO2 to CO2●− radical anion and adsorption of radical anion to surface followed by disproportionate reaction between unreduced CO2 and CO2●− radical anion to form CO32− and CO.
