 | ||
Phosphazene meaning
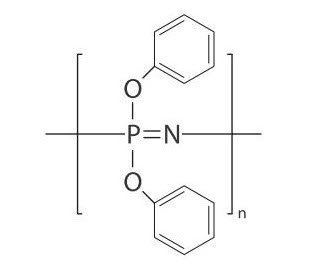
Phosphazenes are a class of chemical compounds in which a phosphorus atom is covalently linked to a nitrogen atom by a double bond and to three other atoms or radicals by single bonds. While other substitutions produce relatively persistent compounds, in organic synthesis the term largely refers to species with three amino substituents bound to phosphorus. The compounds are unusually stable examples of the phosphorane class of molecules and have a remarkable proton affinity. As such, they are one of the eminent examples of neutral, organic superbases. Two examples are hexachlorocyclotriphosphazene and bis(triphenylphosphine)iminium chloride. Phosphazenes are also known as iminophosphoranes and phosphine imides.
Contents
The corresponding polymers are polyphosphazenes. Phosphinimide ligands can be used in catalysis.
Phosphazene bases
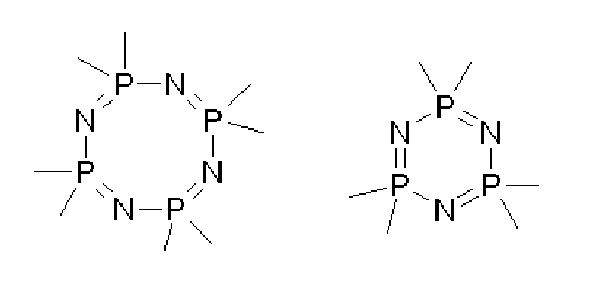
Phosphazene bases are strong non-metallic non-ionic and low-nucleophilic bases. They are stronger bases than regular amine or amidine bases such as Hünig's base or DBU. Protonation takes place at a doubly bonded nitrogen atom. Related to phosphazene bases are the proazaphosphatrane bases, which have a saturated P(NR)3 structure and protonate at phosphorus.
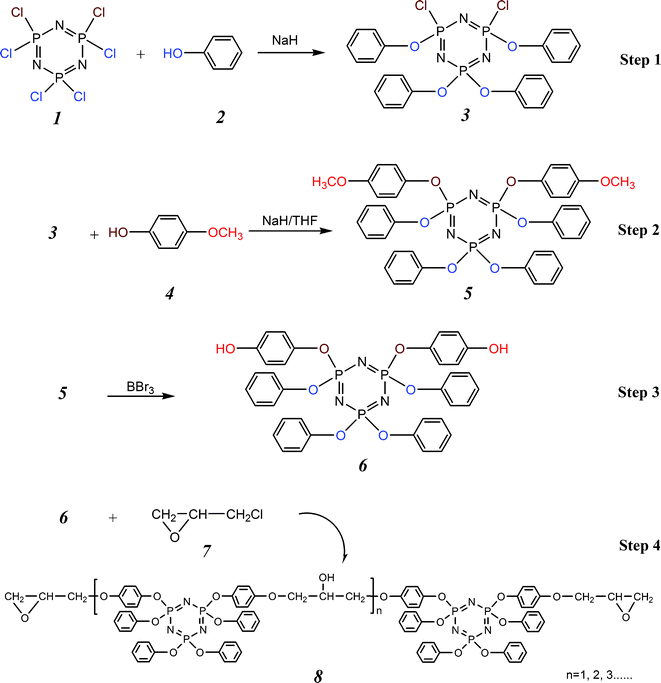
Though the simplest phosphazene superbase, P1-Me, was first synthesized in 1975, chemists assumed that the compounds were highly unstable, like their alkyl-substituted derivatives. The species was regarded at that time as little more than an academic curiosity.
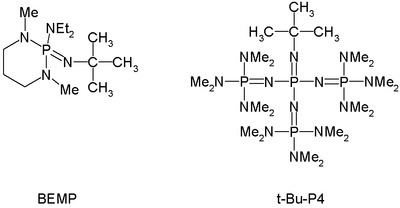
By now phosphazene bases are established reagents in organic synthesis. Perhaps the best known phosphazene bases are BEMP with an acetonitrile pKa of the conjugate acid of 27.6 and the phosphorimidic triamide t-Bu-P4 (pKBH+ = 42.7) also known as Schwesinger base after one of its inventors.
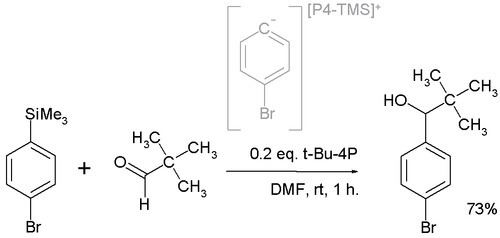
In one application t-Bu-P4 is employed in a nucleophilic addition converting the pivaldehyde to the alcohol:
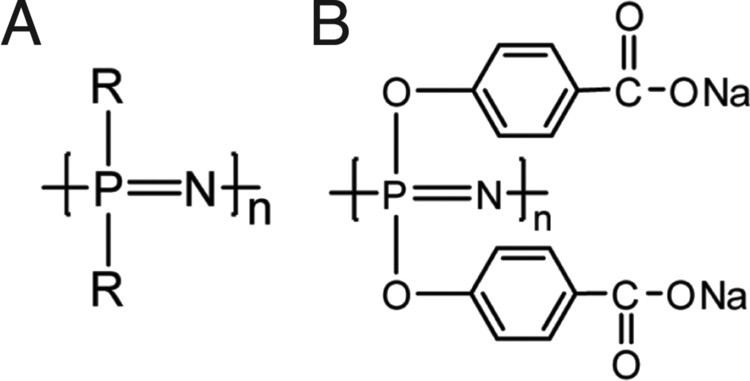
The active nucleophile is believed to be a highly reactive phosphazenium species with full negative charge on the arene sp2 carbon.
Besides organic synthesis, phosphazene bases are used as basic titrants in non-aqueous acid-base titration. Their advantages for this are: they are very strong bases in many solvents and their conjugate acids are inert and non-HBD cations.