Formula C12H8N2 Density 1.31 g/cm³ | Molar mass 180.21 g/mol Appearance colourless crystals | |
 | ||
Related compounds | ||
Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. It is used as a ligand in coordination chemistry, it forms strong complexes with most metal ions.
Contents
- Synthesis
- Coordination chemistry
- Bioinorganic chemistry
- Related phen ligands
- As an indicator for alkyllithium reagents
- References
Synthesis
Phenanthroline may be prepared by two successive Skraup reactions of glycerol with o-phenylenediamine, catalyzed by sulfuric acid, and an oxidizing agent, traditionally aqueous arsenic acid or nitrobenzene. Dehydration of glycerol gives acrolein which condenses with the amine followed by a cyclization.
Coordination chemistry
In terms of its coordination properties, phen is similar to 2,2'-bipyridine (bipy) but binds metals more tightly since the chelating nitrogen donors are preorganized.
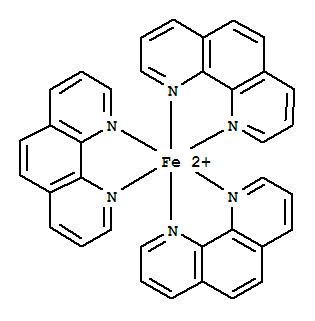
Many homoleptic complexes are known. Particularly well studied is [Fe(phen)3]2+, called "ferroin." It was used for the photometric determination of Fe(II). It is used as a redox indicator with standard potential +1.06 V. The reduced ferrous form has a deep red colour and the oxidised form is light-blue. The pink complex [Ni(phen)3]2+ has been resolved into its Δ and Λ isomers. Copper(I) forms [Cu(phen)2]+, which is luminescent.
Bioinorganic chemistry
The ferroin analogue [Ru(phen)3]2+ has long been known to be bioactive.
1,10-Phenanthroline is an inhibitor of metallopeptidases, with one of the first observed instances reported in carboxypeptidase A. Inhibition of the enzyme occurs by removal and chelation of the metal ion required for catalytic activity, leaving an inactive apoenzyme. 1,10-Phenanthroline targets mainly zinc metallopeptidases, with a much lower affinity for calcium.
Related phen ligands
A variety of substituted derivatives of phen have been examined as ligands. Neocuproine, 2,9-dimethyl-1,10-phenanthroline, is a bulky ligand. In "bathophenanthroline," the 4 and 7 positions are substituted by phenyl groups. The more electron-rich phenanthroline ligand is 3,4,7,8-tetramethyl-1,10-phenanthroline.
As an indicator for alkyllithium reagents
Alkyllithium reagents form deeply colored derivatives with phenanthroline. The alkyllithium content of solutions can be determined by treatment of such reagents with small amounts of phenanthroline (ca. 1 mg) followed by titration with alcohols to a colourless endpoint. Grignard reagents may be similarly titrated.
