 | ||
Organorhenium chemistry describes the compounds with Re-C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.
Contents
General features
Re exists in ten known oxidation states from −3 to +7 except −2, and all but Re(−3) are represented by organorhenium compounds. Most are prepared from salts of perrhenate and related binary oxides. The halides, e.g., ReCl5 are also useful precursors as are certain oxychlorides.
A noteworthy feature of organorhenium chemistry is the coexistence of oxide and organic ligands in the same coordination sphere.
Carbonyl compounds
Dirhenium decacarbonyl is a common entry point to other rhenium carbonyls. The general patterns are similar to the related manganese carbonyls. It is possible to reduce this dimer with sodium amalgam to Na[Re(CO)5] with rhenium in the formal oxidation state −1. Bromination of dirhenium decacarbonyl gives bromopentacarbonylrhenium(I), then reduced with zinc and acetic acid to pentacarbonylhydridorhenium:
Re2(CO)10 + Br2 → 2 Re(CO)5BrRe(CO)5Br + Zn + HOAc → Re(CO)5H + ZnBr(OAc)Bromopentacarbonylrhenium(I) is readily decarbonylated. In refluxing water, it forms the triaquo cation:
Re(CO)5Br + 3 H2O → [Re(CO)3(H2O)3]Br + 2 COWith tetraethylammonium bromide Re(CO)5Br reacts to give the anionic tribromide:
Re(CO)5Br + 2 NEt4Br → [NEt4]2[Re(CO)3Br3] + 2 COCyclopentadienyl complexes
One of the first transition metal hydride complexes to be reported was (C5H5)2ReH. A variety of half-sandwich compounds have been prepared from (C5H5)Re(CO)3 and (C5Me5)Re(CO)3. Notable derivatives include the electron-precise oxide (C5Me5)ReO3 and (C5H5)2Re2(CO)4.
Re-alkyl and aryl compounds
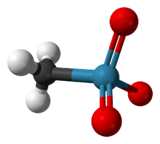
Rhenium forms a variety of alkyl and aryl derivatives, often with pi-donor coligands such as oxide. Well known is methylrhenium trioxide ("MTO"), CH3ReO3 a volatile, colourless solid. This compound has been used as a catalyst in some laboratory experiments. It can be prepared by many routes, a typical method is the reaction of Re2O7 and tetramethyltin:
Re2O7 + (CH3)4Sn → CH3ReO3 + (CH3)3SnOReO3Analogous alkyl and aryl derivatives are known. MTO catalyses for the oxidations with hydrogen peroxide. Terminal alkynes yield the corresponding acid or ester, internal alkynes yield diketones, and alkenes give epoxides. MTO also catalyses the conversion of aldehydes and diazoalkanes into an alkene.
Rhenium is also able to make complexes with fullerene ligands such as Re2(PMe3)4H8(η2:η2C60).
