Abbreviations NOF Boiling point -72.4 °C Melting point -166 °C Appearance Colourless gas | Formula NOF Molar mass 49.0045 g/mol Density 1.72 g/cm³ | |
 | ||
Related compounds | ||
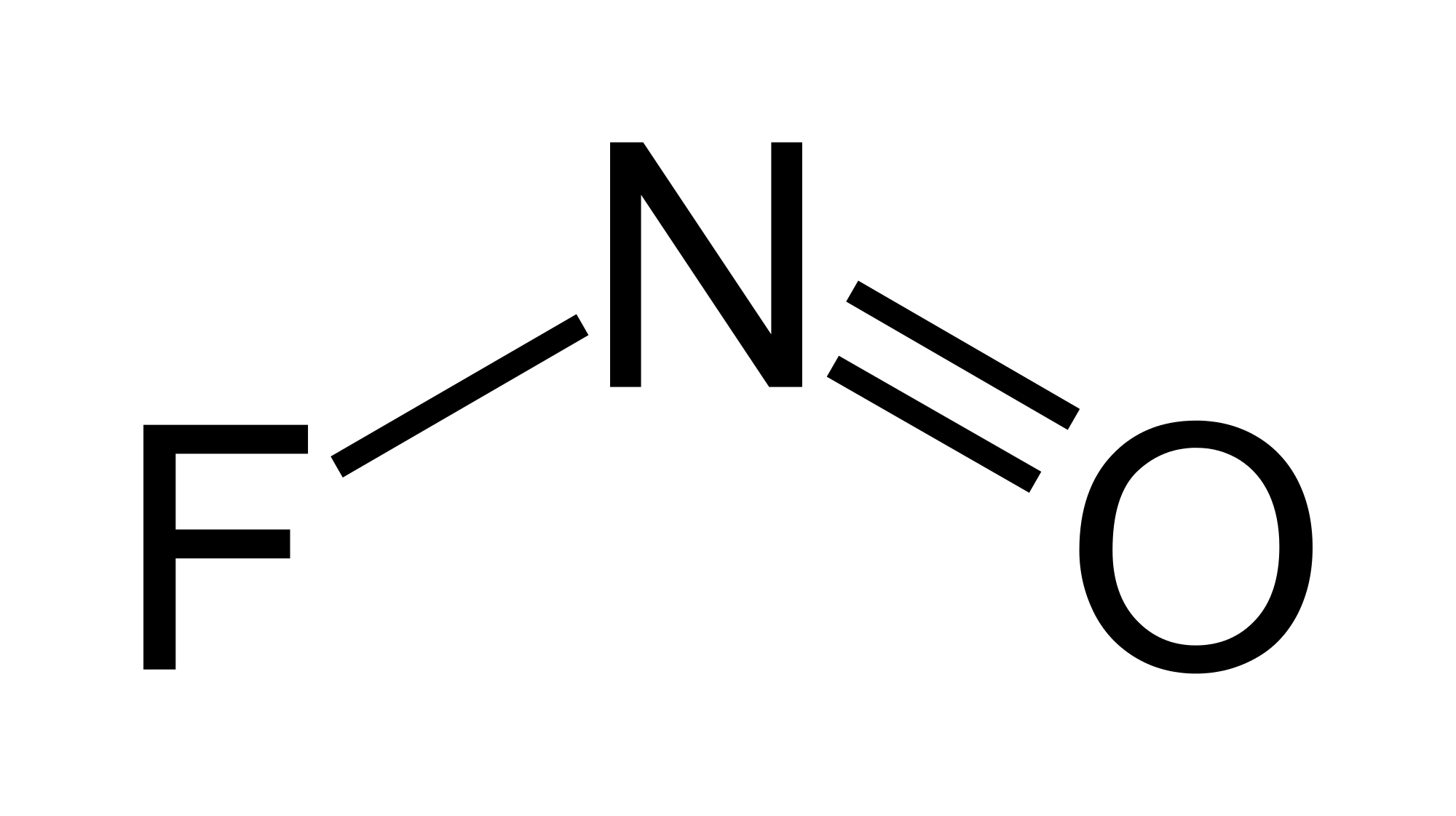
Nitrosyl fluoride lewis structure dot diagram
Nitrosyl fluoride, NOF, is a covalently bonded nitrosyl compound.
Contents
Reactions
NOF is a highly reactive fluorinating agent that converts many metals to their fluorides, releasing nitric oxide in the process:
n NOF + M → MFn + n NONOF also fluorinates fluorides to form adducts that have a salt-like character, such as NOBF4.
Aqueous solutions of NOF are powerful solvents for metals, by a mechanism similar to that seen in aqua regia. Nitrosyl fluoride reacts with water to form nitrous acid, which then forms nitric acid:
NOF + H2O → HNO2 + HF3 HNO2 → HNO3 + 2 NO + H2ONitrosyl fluoride can also convert alcohols to nitrites:
ROH + NOF → RONO + HFIt has a bent molecular shape.
Uses
Nitrosyl fluoride is used as a solvent and as a fluorinating and nitrating agent in organic synthesis. It has also been proposed as an oxidizer in rocket propellants.
References
Nitrosyl fluoride Wikipedia(Text) CC BY-SA
