 | ||
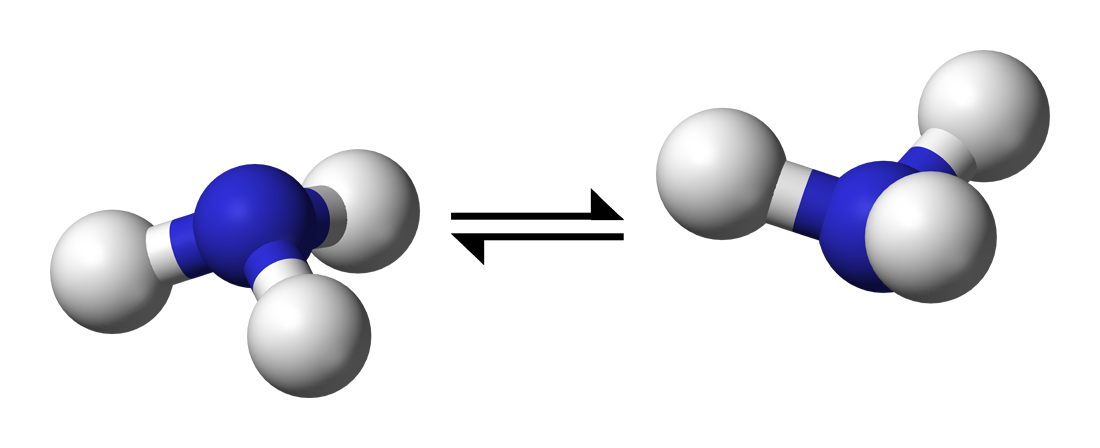
In chemistry, nitrogen inversion is a fluxional process in compounds with a nitrogen atom that has a pyramidal geometry, such as ammonia (NH3), whereby the molecule "turns inside out". It is a rapid oscillation of the nitrogen atom from one side of the plane formed by the substituents to the other side, passing through a planar transition state. For a compound that would otherwise be chiral due to a nitrogen stereocenter, nitrogen inversion allows its enantiomers to rapidly interconvert, making chiral resolution impossible unless the inversion process is prevented by steric or electronic effects. The concept of nitrogen inversion can be extended to other compounds that contain atoms of other elements with trigonal pyramidal geometry, such as carbanions, phosphines, arsines, stibines and sulfoxides, in which case it is called pyramidal inversion.
Contents
Energy barrier considerations
The ammonia interconversion is rapid at room temperature. Two factors contribute to the rapidity of the inversion: a low energy barrier (24.2 kJ/mol; 5.8 kcal/mol) and a narrow width of the barrier itself, which allows for frequent quantum tunnelling (see below). In contrast, phosphine (PH3) inverts very slowly at room temperature (energy barrier: 132 kJ/mol).
Consequences for optical isomerism
Amines of the type RR′R"N and RR′NH are chiral, but they typically cannot be obtained as individual enantiomers because of the rapidity of the nitrogen inversion. The situation is very different for ammonium salts, such as RR′R″HN+ and RR′R″R‴N+, and amine oxides, such as RR′HNO and RR′R″NO, which are optically stable. The barrier to pyramidal inversion in these compounds is much higher because they have four covalent bonds rather than any nonbonded electron pairs, so their enantiomers do not rapidly interconvert and are therefore separable. Unlike amines, the corresponding chiral phosphines (RR′R″P and RR′PH), sulfonium salts (RR′R″S+), and sulfoxides (RR′SO) are also optically stable even though they have a non-bonded pair.
Quantum effects
Ammonia exhibits a quantum tunnelling due to a narrow tunneling barrier, and not due to thermal excitation. Superposition of two states leads to energy level splitting, which is used in ammonia masers.
Conditions
For nitrogen inversion to occur:
Examples
The inversion of ammonia was first detected by microwave spectroscopy in 1934.
In one study the inversion in an aziridine was slowed by a factor of 50 by placing the nitrogen atom in the vicinity of a phenolic alcohol group compared to the oxidized hydroquinone
The system interconverts by oxidation by oxygen and reduction by sodium dithionite.
