 | ||
N-Sulfinyl imines (N-sulfinylimines, sulfinimines, thiooxime S-oxides) are a class of imines bearing a sulfinyl group attached to nitrogen. These imines display usefully stereoselectivity reactivity and due to the presence of the chiral electron withdrawing N-sulfinyl group. They allow 1,2-addition of organometallic reagents to imines. The N-sulfinyl group exerts powerful and predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center is prevented because anions are stabilized at nitrogen (i.e., the sulfinyl group is a versatile amine protection group). The sulfinyl chiral auxiliary is readily removed by simple acid hydrolysis. The addition of organometallic reagents to N-sulfinyl imines is the most reliable and versatile method for the asymmetric synthesis of amine derivatives. These building blocks have been employed in the asymmetric synthesis of numerous biologically active compounds.
Contents
Synthesis
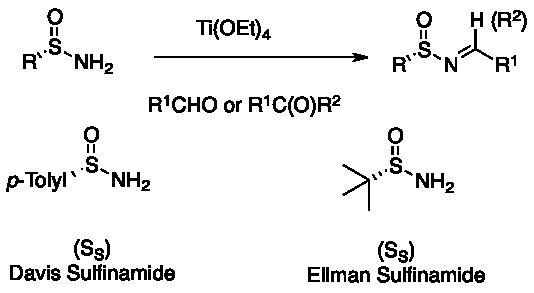
The first N-sulfinyl imines in racemic form were reported by Franklin A. Davis in 1974 by oxidation of p-toluene-sulfenyl imines with m-CPBA. Enantiopure p-toluene-sulfinyl imines, described by M. Cinquini et al. in 1977, involve the reaction of the commercially available Andersen reagent (menthyl p-toluenesulfinate) with metallo-ketimines but is limited to ketone derived N-sulfinyl imines. A more general method for the preparation of N-sulfinyl imines is the asymmetric oxidation of achiral sulfenyl-imines with a chiral oxaziridine reported by Davis et al. in 1992. While this method played an important role in initiating chiral N-sulfinyl imine chemistry, it utility is limited by the difficulty of preparation of the N-sulfonyloxaziridne. More practical is the one-pot procedure from the Andersen reagent making a variety of p-toluene-sulfinyl imines availabled from both aromatic and aliphatic aldehydes.
The most widely used method for the asymmetric synthesis of N-sulfinyl imines is the condensation of enantiopure primary sulfinamides with aldehyde or ketones. A mild Lewis acid dehydrating reagents such as Ti(OEt)4, as well as other desiccants/water scavengers facilitate the condensation. Many sulfinamides are commercially available in both (R)- and (S)-forms. The two most commonly used are the Davis p-toluene-sulfinamide and the Ellman tert-butanesulfinamide
Applications
The p-toluene-sulfinyl imines are useful for the highly diastereoselective asymmetric synthesis of α-amino acids, β-amino acids, syn- and anti-2,3-diamino esters, α-amino aldehydes and ketones, β-amino ketones, α-amino phosphonates, aziridine 2-carboxylates, and aziridine 2-phosphnates. Many of these same transformations can been carried out with tert-butylsulfinyl imines. For the asymmetric synthesis of amines tert-butane-sulfinyl imines are required because lithium and Grignard reagents react at the sulfinyl sulfur in p-toluene-sulfinyl imines. Mild acid treatment readily removes the N-sulfinyl group in the sulfinamide products affording the free amine derivatives. An advantage of tert-butytsulfinyl imines is that acid treatment of the corresponding sulfinamides leads to easily removal by-products.
