 | ||
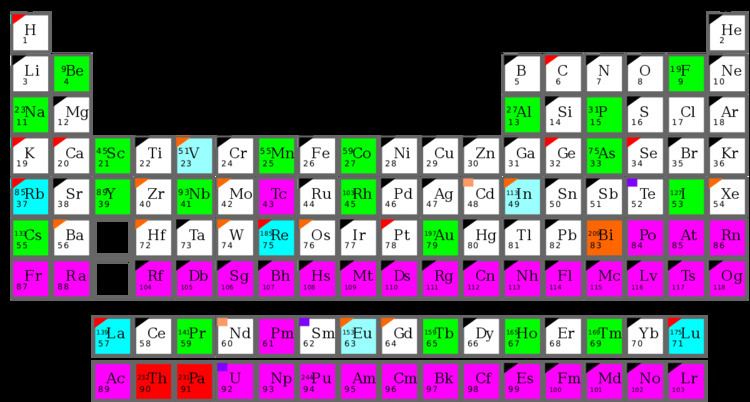
A mononuclidic element is one of the 22 chemical elements that is found naturally on Earth essentially as a single nuclide (which may, or may not, be a stable nuclide). This single nuclide will have a characteristic atomic mass. Thus, the element's natural isotopic abundance is dominated either by one stable isotope or by one very long-lived isotope. There are 19 elements in the first category (which are both monoisotopic and mononuclidic), and 3 (bismuth, thorium and protactinium) in the second category (mononuclidic but not monoisotopic, since they have zero, not one, stable nuclides). A list of the 22 mononuclidic elements is given at the end of this article.
Contents
- Use in metrology
- Contamination by unstable trace isotopes
- Complete list of the 22 mononuclidic elements
- References
Of the 26 monoisotopic elements that, by definition, have only one stable isotope, there exist 7 (26 minus 19 = 7) which are nevertheless not considered mononuclidic, due to the presence of a significant fraction of a very long-lived (primordial) radioisotope occurring in their natural abundance. These elements are vanadium, rubidium, indium, lanthanum, europium, rhenium, and lutetium.
Use in metrology
Mononuclidic elements are of scientific importance because their atomic weights can be measured to high accuracy, since there is minimal uncertainty associated with the isotopic abundances present in a given sample. Another way of stating this, is that, for these elements, the relative atomic mass and atomic mass are the same.
In practice, only 11 of the mononuclidic elements are used in standard atomic weight metrology. These are aluminium, bismuth, caesium, cobalt, gold, manganese, phosphorus, scandium, sodium, terbium, and thorium.
Contamination by unstable trace isotopes
Trace concentrations of unstable isotopes of some mononuclidic elements are found in natural samples. For example, beryllium-10 (10Be), with a half-life of 1.4 million years, is produced by cosmic rays in the Earth's upper atmosphere; iodine-129 (129I), with a half-life of 15.7 million years, is produced by various cosmogenic and nuclear mechanisms; 137Cs, with a half-life of 30 years, is generated by nuclear fission. Such isotopes are used in a variety of analytical and forensic applications.
Complete list of the 22 mononuclidic elements
Data from Atomic Weights and Isotopic Compositions ed. J. S. Coursey, D. J. Schwab and R. A. Dragoset, National Institute of Standards and Technology (2005).
