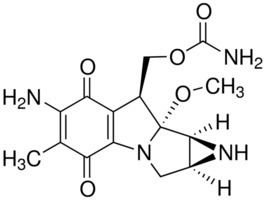
Formula C15H18N4O5 Molar mass 334.33 g/mol | CAS ID 50-07-7 Melting point 360 °C | |
 | ||
IUPAC ID [6-Amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl carbamate | ||
The mechanism of mitomycin c
The mitomycins are a family of aziridine-containing natural products isolated from Streptomyces caespitosus or Streptomyces lavendulae. They include mitomycin A, mitomycin B, and mitomycin C. When the name mitomycin occurs alone, it usually refers to mitomycin C; it is the international nonproprietary name for mitomycin C.
Contents
- The mechanism of mitomycin c
- Anticancer chemotherapy mitomycin c part 1
- Biosynthesis
- Biological effects
- References
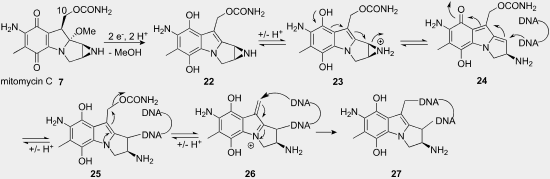
Anticancer chemotherapy mitomycin c part 1
Biosynthesis

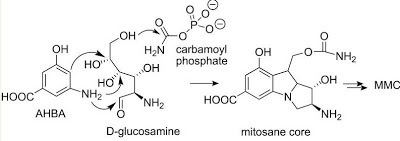
In general, the biosynthesis of all mitomycins proceeds via combination of 3-amino-5-hydroxybenzoic acid (AHBA), D-glucosamine, and carbamoyl phosphate, to form the mitosane core, followed by specific tailoring steps. The key intermediate, AHBA, is a common precursor to other anticancer drugs, such as rifamycin and ansamycin.

Specifically, the biosynthesis begins with the addition of phosphoenolpyruvate (PEP) to erythrose-4-phosphate (E4P) with a yet undiscovered enzyme, which is then ammoniated to give 4-amino-3-deoxy-D-arabino heptulosonic acid-7-phosphate (aminoDHAP). Next, DHQ synthase catalyzes a ring closure to give 4-amino3-dehydroquinate (aminoDHQ), which is then undergoes a double oxidation via aminoDHQ dehydratase to give 4-amino-dehydroshikimate (aminoDHS). The key intermediate, 3-amino-5-hydroxybenzoic acid (AHBA), is made via aromatization by AHBA synthase.
Synthesis of the key intermediate, 3-amino-5-hydroxy-benzoic acid.

The mitosane core is synthesized as shown below via condensation of AHBA and D-glucosamine, although no specific enzyme has been characterized that mediates this transformation. Once this condensation has occurred, the mitosane core is tailored by a variety of enzymes. Both the sequence and the identity of these steps are yet to be determined.
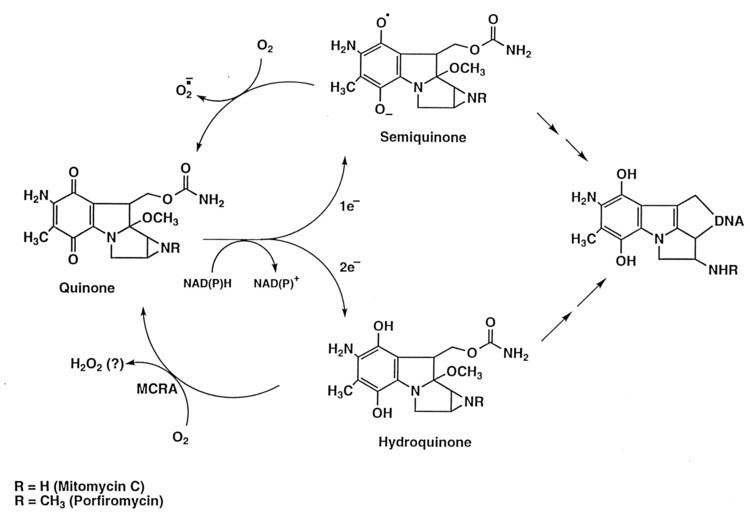
Biological effects
In the bacterium Legionella pneumophila, mitomycin C induces competence for transformation. Natural transformation is a process of DNA transfer between cells, and is regarded as a form of bacterial sexual interaction. In the fruit fly Drosophila melanogaster, exposure to mitomycin C increases recombination during meiosis, a key stage of the sexual cycle. In the plant Arabidopsis thaliana, mutant strains defective in genes necessary for recombination during meiosis and mitosis are hypersensitive to killing by mitomycin C. It has been suggested that these, and other related findings, can be explained by the idea that during sexual processes in prokaryotes (transformation) and eukaryotes (meiosis) DNA crosslinks and other damages introduced by mitomycin C are removed by recombinational repair.
Mitomycin C has recently been found to have very good activity against stationary phase and against persisters created by Borrelia burgdorferi, the causative agent of lyme disease.
