Appearance white solid | ||
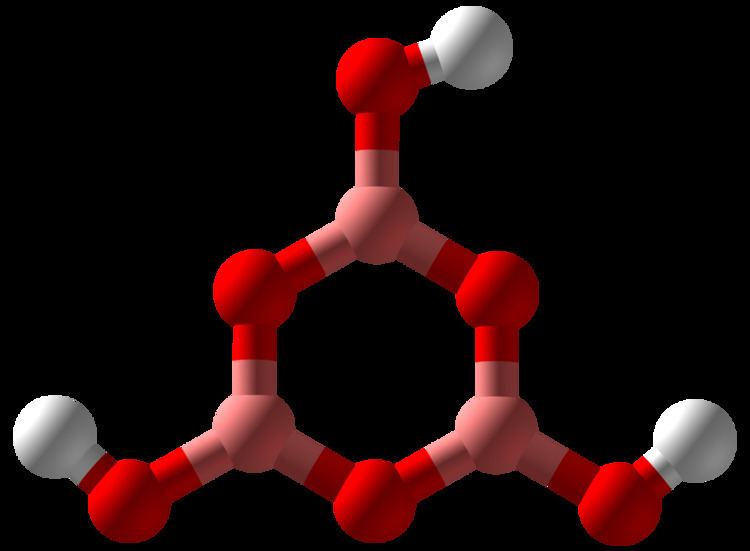 | ||
Metaboric acid is the name for a family of inorganic compounds formed by the dehydration of boric acid. Metaboric acids are colourless solids with the empirical formula HBO2. There are three forms of metaboric acid, all are white solids. One form of metaboric acid is molecule, and the other forms are polymers.
Contents
Preparation
Heating of boric acid at 80-100 °C releases water to give orthorhombic metaboric acid: 3 B(OH)3 → (BOH)3O3 + 3 H2O
This form is molecular, consisting of discrete trimers. This molecule has C3h symmetry and forms a sheet-like structure, similar to that of boric acid itself. It is also called "modification III" of the metaboric acids.
Upon heating at 130-140 °C in a sealed ampoule (to prevent dehydration), orthorhombic metaboric acid converts to the monoclinic form:
(BOH)3O3 → B3O4(OH)(H2O)This material, called modification II, has a polymeric structure, and a higher melting point (201 °C) and density (2.045 g/cm3). The structure of this species resembles its precursor except that the rings are connected and 1/3 of the boron centres are tetrahedral.
Above 140 °C, boric acid or the other forms of metaboric acid convert to cubic metaboric acid.
Metaborates
Metaborates are derivatives of BO2−. Like metaboric acid, the metaborates exist with disparate structures. Examples are sodium and potassium metaborates, salts formed by deprotonation of orthorhombic metaboric acid containing the cyclic B3O63− ion and calcium metaborate, Ca(BO2)2, which contains the chain polymeric ion (BO2−)n.
