Formula Mg(NO3)2 Melting point 88.9 °C Density 2.3 g/cm³ | Molar mass 148.3 g/mol Boiling point 330 °C Appearance White crystalline solid | |
 | ||
Magnesium nitrate is a hygroscopic salt with the formula Mg(NO3)2. In air, it quickly forms the hexahydrate with the formula Mg(NO3)2·6H2O (and molar weight of 256.41 g/mol). It is very soluble in both water and ethanol.
Contents

Uses

Magnesium nitrate occurs in mines and caverns as nitromagnesite (hexahydrate form). This form is not common, although it may be present where guano contacts magnesium-rich rock. It is used in the ceramics, printing, chemical and agriculture industries. Its fertilizer grade has 10.5% nitrogen and 9.4% magnesium, so it is listed as 10.5-0-0 + 9.4% Mg. Fertilizer blends containing magnesium nitrate usually have ammonium nitrate, calcium nitrate, potassium nitrate and micronutrients; these blends are used in the greenhouse and hydroponics trade.
Production
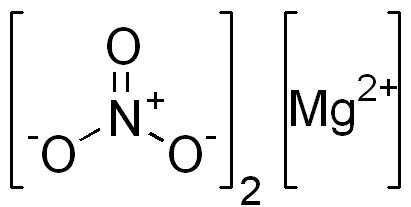
The magnesium nitrate used in commerce is a man-made product. It can be synthesized in a variety of ways. The reaction between nitric acid and magnesium metal
2 10HNO3 + 4Mg = 4Mg(NO3)2 + NH4NO3 + 3H2Oresults in magnesium nitrate.
Magnesium hydroxide and ammonium nitrate also react to form magnesium nitrate as ammonia is released as a by-product.
Mg(OH)2 + 2 NH4NO3 → Mg(NO3)2 + 2 NH3 + 2 H2OReactions

Magnesium nitrate reacts with alkali metal hydroxide to form the corresponding nitrate: Mg(NO3)2 + 2 NaOH → Mg(OH)2 + 2 NaNO3.

Since magnesium nitrate has a high affinity for water, heating the hexahydrate does not result in the dehydration of the salt, but rather its decomposition into magnesium oxide, oxygen, and nitrogen oxides: 2 Mg(NO3)2 → 2 MgO + 4 NO2 + O2. The absorption of these nitrogen oxides in water is one possible route to synthesize nitric acid. Although inefficient, this method does not require the use of any strong acid.
Anhydrous magnesium nitrate is also used to increase the concentration of nitric acid past its azeotrope of approximately 68% nitric acid and 32% water. It is also occasionally used as a desiccant.
