 | ||
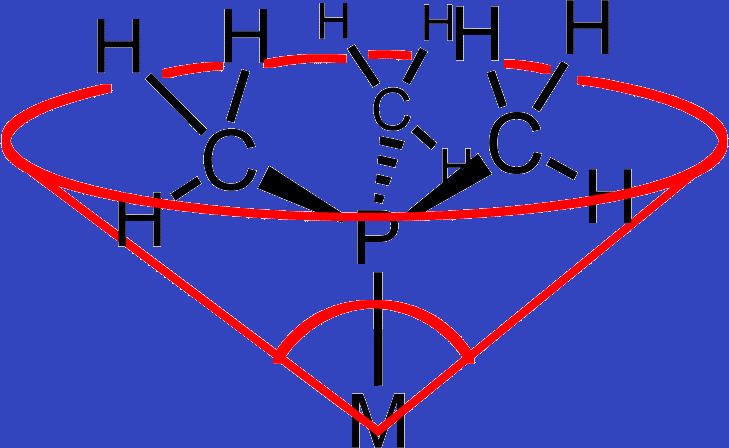
The ligand cone angle (a common example being the Tolman cone angle or θ) is a measure of the size of a ligand. It is defined as the solid angle formed with the metal at the vertex and the hydrogen atoms at the perimeter of the cone (see figure). Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any ligand. The term cone angle was introduced by Chadwick A. Tolman, a research chemist at Dupont. Originally applied to phosphines, the cone angles were originally determined by taking measurements from accurate physical models of them.
Contents
Asymmetric cases
The concept of cone angle is most easily visualized with symmetrical ligands, e.g. PR3. But the approach has been refined to include less symmetrical ligands of the type PRR′R″ as well as diphosphines. In such asymmetric cases, the substituent angles' half angles, θi/2, are averaged and then doubled to find the total cone angle, θ. In the case of diphosphines, the θi/2 of the backbone is approximated as half the chelate bite angle, assuming a bite angle of 74°, 85°, and 90° for diphosphines with methylene, ethylene, and propylene backbones, respectively. The Manz cone angle is often easier to compute than the Tolman cone angle:
Variations
The Tolman cone angle method assumes empirical bond data and defines the perimeter as the maximum possible circumscription of an idealized free-spinning substituent. In contrast, the solid-angle concept derives both bond length and the perimeter from empirical solid state crystal structures. There are advantages to each system.
If the geometry of a ligand is known, either through crystallography or computations, an exact cone angle (θ) can be calculated. No assumptions about the geometry are made, unlike the Tolman method.
Application
The concept of cone angle is of practical importance in homogeneous catalysis because the size of the ligand affects the reactivity of the attached metal center. In a famous example, the selectivity of hydroformylation catalysts is strongly influenced by the size of the coligands. Despite being monovalent, some phosphines are large enough to occupy more than half of the coordination sphere of a metal center.
