Trade names Sporanox, Orungal MedlinePlus a692049 CAS ID 84625-61-6 Protein binding 99.8% | AHFS/Drugs.com Monograph ATC code J02AC02 (WHO) Molar mass 705.64 g/mol | |
 | ||
Pregnancycategory AU: B3US: C (Risk not ruled out) Routes ofadministration Oral (capsules, oral solution), local (vaginal suppository), IV; Oral only (UK and US) | ||
Itraconazole (code name R51211), invented in 1984, is a triazole antifungal agent prescribed to patients with fungal infections. The drug may be given orally or intravenously.
Contents
Medical uses
Itraconazole has a broader spectrum of activity than fluconazole (but not as broad as voriconazole or posaconazole). In particular, it is active against Aspergillus, which fluconazole is not. It is also licensed for use in blastomycosis, sporotrichosis, histoplasmosis, and onychomycosis. Itraconazole is over 99% protein-bound and has virtually no penetration into cerebrospinal fluid. Therefore, it should never be used to treat meningitis or other central nervous system infections. According to the Johns Hopkins Abx Guide, it has "negligible CSF penetration, however treatment has been successful for cryptococcal and coccidioidal meningitis".
It is also prescribed for systemic infections, such as aspergillosis, candidiasis, and cryptococcosis, where other antifungal drugs are inappropriate or ineffective.
Itraconazole has also recently been explored as an anticancer agent for patients with basal cell carcinoma, non-small cell lung cancer, and prostate cancer. For example, in a phase II study involving men with advanced prostate cancer, high-dose itraconazole (600 mg/day) was associated with significant PSA responses and a delay in tumor progression. Itraconazole also showed activity in a phase II trial in men with non-small cell lung cancer when it was combined with the chemotherapy agent, pemetrexed.
Available forms
Itraconazole is produced as blue 22 mm (0.87 in) capsules with tiny 1.5 mm (0.059 in) blue pellets inside. Each capsule contains 100 mg and is usually taken twice a day at twelve-hour intervals. The Sporanox brand of itraconazole has been developed and marketed by Janssen Pharmaceutica, a subsidiary of Johnson & Johnson. The three-layer structure of these blue capsules is complex because itraconazole is insoluble and is sensitive to pH. The complicated procedure not only requires a specialized machine to create it, but also the method used has manufacturing problems. Also, the pill is quite large, making it difficult for many patients to swallow. Parts of the processes of creating Sporanox were discovered by the Korean Patent Laid-open No. 10-2001-2590. The tiny blue pellets contained in the capsule are manufactured in Beerse, Belgium.
The intravenous preparation is no longer available in the US as of October 11, 2007 per Ortho-Biotech Professional Letter but may be available in other countries.
Conventional itraconazole (e.g. Sporanox) has relatively low bioavailability after oral administration, especially when given in capsule form on an empty stomach. The capsule form is a molecular dispersion of itraconazole in amorphous HPMC polymer. The fast-dissolving polymer targets a supersaturated solution of itraconazole from which enhanced absorption can be expected. Recently, itraconazole was found to contribute to the formation of nanofibers in certain simulated intestinal fluids. These nanofibers have a uniform width of 12 nm and a length up to several micrometers. The oral solution is better absorbed. The cyclodextrin contained in the oral solution can cause an osmotic diarrhea, and if this is a problem, then half the dose can be given as oral solution and half as capsule to reduce the amount of cyclodextrin given. "Sporanox" itraconazole capsules should always be taken with food, as this improves absorption, however the manufacturers of "Lozanoc" assert that it may be taken "without regard to meals". Itraconazole oral solution should be taken an hour before food, or two hours after food (and likewise if a combination of capsules and oral solution are used). Itraconazole may be taken with orange juice or cola, as absorption is also improved by acid. Absorption of itraconazole is impaired when taken with an antacid, H2 blocker or proton pump inhibitor.
Adverse effects
Itraconazole is a relatively well-tolerated drug (although not as well tolerated as fluconazole or voriconazole) and the range of adverse effects it produces is similar to the other azole antifungals:
The cyclodextrin used to make the syrup preparation can cause diarrhea. Side effects that may indicate a greater problem include:
Interactions
The following drugs should not be taken with itraconazole:
Mechanism of action
The mechanism of action of itraconazole is the same as the other azole antifungals: it inhibits the fungal-mediated synthesis of ergosterol, via inhibition of lanosterol 14α-demethylase. Because of its ability to inhibit cytochrome P450 3A4 CC-3, caution should be used when considering interactions with other medications.
Itraconazole is pharmacologically distinct from other azole antifungal agents in that it is the only inhibitor in this class that has been shown to inhibit both the hedgehog signaling pathway and angiogenesis. These distinct activities are unrelated to inhibition of the cytochrome P450 lanosterol 14 alpha-demethylase and the exact molecular targets responsible remain unidentified. Functionally, the antiangiogenic activity of itraconazole has been shown to be linked to inhibition of glycosylation, VEGFR2 phosphorylation, trafficking, and cholesterol biosynthesis pathways. Evidence suggests the structural determinants for inhibition of hedgehog signaling by itraconazole are recognizably different from those associated with antiangiogenic activity.
Pharmacokinetics
Itraconazole, like cyclosporine, quinidine and clarithromycin, can inhibit P-glycoproteins causing drug-drug interactions by reducing elimination and increasing absorption of organic cation drugs. With conventional Itraconazole preparations serum levels can vary greatly between patients, often resulting in serum concentrations lower than the therapeutic index. It has therefore been conventionally advised that patients take itraconazole after a fatty meal rather than prior to eating.
A product (Lozanoc) licensed through the European union decentralised procedure has increased bioavailability, decreased sensitivity to co ingestion of food, and hence decreased variability of serum levels.
Anti-cancer properties
Itraconazole has been investigated as an anti-cancer agent. A small randomized clinical trial found that patient survival was increased when itraconazole was added to pemetrexed. Median survival for the patients in the study who received both itraconazole and pemetrexed was 32 months, while median survival for patients who only received pemetrexed was 8 months.
Chemistry
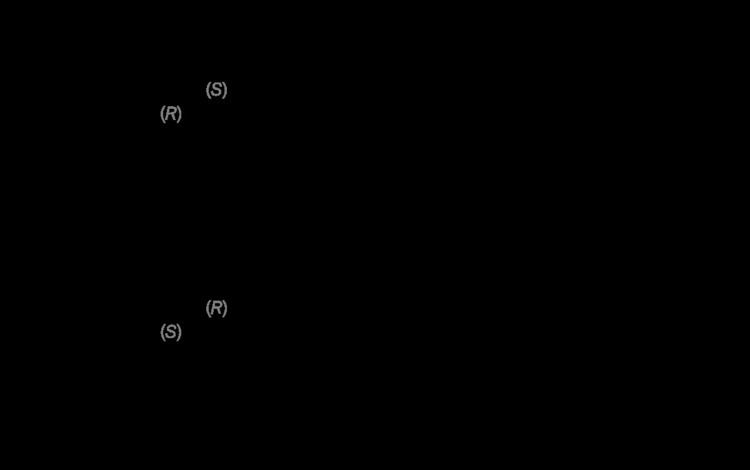
Itraconazole molecule has three chiral carbons. The two chiral centers in the dioxolane ring are fixed in relation to one another, and the triazolomethylene and aryloxymethylene dioxolane-ring substituents are always cis to each other. The clinical formulation is a 1:1:1:1 mixture of four stereoisomers (two enantiomeric pairs).
