Species Human Entrez 3552 | Human Mouse Ensembl ENSG00000115008 | |
 | ||
Aliases IL1A, IL-1A, IL1, IL1-ALPHA, IL1F1, interleukin 1 alpha External IDs OMIM: 147760 MGI: 96542 HomoloGene: 480 GeneCards: IL1A | ||
Interleukin 1 alpha (IL-1α) also known as hematopoietin 1 is a cytokine of the interleukin 1 family that in humans is encoded by the IL1A gene. In general, Interleukin 1 is responsible for the production of inflammation, as well as the promotion of fever and sepsis. IL-1α inhibitors are being developed to interrupt those processes and treat diseases.
Contents
- Discovery
- Alternative names
- Synthesis and structure
- Interactions
- Regulatory molecules
- In vitro
- In vivo
- Pharmaceutical
- References
IL-1α is produced mainly by activated macrophages, as well as neutrophils, epithelial cells, and endothelial cells. It possesses metabolic, physiological, haematopoietic activities, and plays one of the central roles in the regulation of the immune responses. It binds to the interleukin-1 receptor. It is on the pathway that activates tumor necrosis factor-alpha.
Discovery
Interleukin 1 was discovered by Gery in 1972. He named it lymphocyte-activating factor (LAF) because it was a lymphocyte mitogen. It was not until 1985 that interleukin 1 was discovered to consist of two distinct proteins, now called interleukin-1 alpha and interleukin-1 beta.
Alternative names
IL-1α is also known as fibroblast-activating factor (FAF), lymphocyte-activating factor (LAF), B-cell-activating factor (BAF), leukocyte endogenous mediator (LEM), epidermal cell-derived thymocyte-activating factor (ETAF), serum amyloid A inducer or hepatocyte-stimulating factor (HSP), catabolin, hemopoetin-1 (H-1), endogenous pyrogen (EP), and proteolysis-inducing factor (PIF).
Synthesis and structure
IL-1α is a unique member in the cytokine family in the sense that the structure of its initially synthesized precursor does not contain a signal peptide fragment (same is known for IL-1β and IL-18). After processing by the removal of N-terminal amino acids by specific proteases, the resulting peptide is called "mature" form. Calpain, a calcium-activated cysteine protease, associated with the plasma membrane, is primarily responsible for the cleavage of the IL-1α precursor into a mature molecule. Both the 31kDa precursor form of IL-1α and its 18kDa mature form are biologically active.
The 31 kDa IL-1α precursor is synthesized in association with cytoskeletal structures (microtubules), unlike most secreted proteins, which are translated on ribosomes associated with rough endoplasmic reticulum.
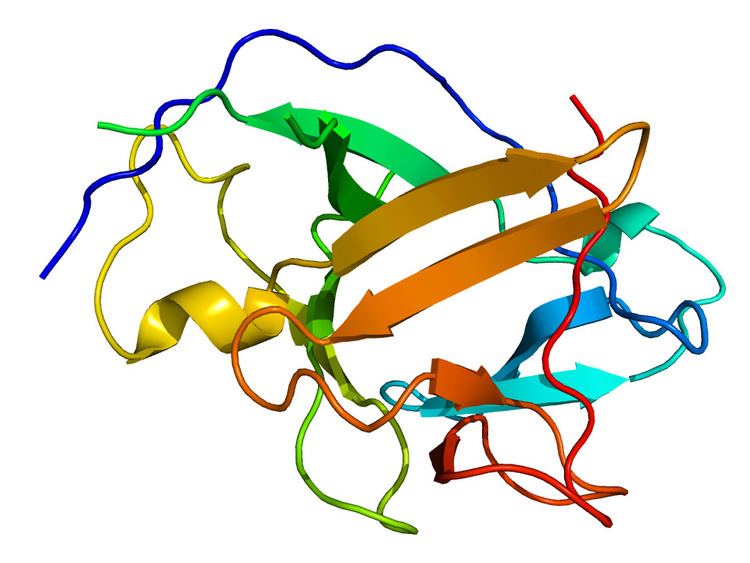
The three-dimensional structure of the IL-1α contains an open-ended barrel composed entirely of beta-pleated strands. Crystal structure analysis of the mature form of IL-1α shows that it has two sites of binding to IL-1 receptor. There is a primary binding site located at the open top of its barrel, which is similar but not identical to that of IL-1β.
Interactions
IL1A has been shown to interact with HAX1, and NDN.
Although there are many interactions of IL-1α with other cytokines, the most consistent and most clinically relevant is its synergism with TNF. IL-1α and TNF are both acute-phase cytokines that act to promote fever and inflammation. There are, in fact, few examples in which the synergism between IL-1α and TNFα has not been demonstrated. These include radioprotection, the Shwartzman reaction, PGE2 synthesis, sickness behavior, nitric oxide production, nerve growth factor synthesis, insulin resistance, loss of mean body mass, and IL-8 and chemokine synthesis.
Regulatory molecules
The most important regulatory molecule for IL-1α activity is IL-1Ra, which is usually produced in a 10- to 100-fold molar excess. In addition, the soluble form of the IL-1R type I has a high affinity for IL-1α and is produced in a 5-10 molar excess. IL-10 also inhibits IL-1α synthesis.
In vitro
IL-1α possesses biological effect on cells in the picomolar to femtomolar range. In particular, IL-1α:
In vivo
Shortly after an onset of an infection into organism, IL-1α activates a set of immune system response processes. In particular, IL-1α:
Topically administered IL-1α also stimulates expression of FGF and EGF, and subsequent fibroblasts and keratinocytes proliferation. This, plus the presence of large depot of IL-1α precursor in keratinocytes, suggests that locally released IL-1α may play an important role and accelerate wound healing.
IL-1α is known to protect against lethal doses of γ-irradiation in mice, possibly as a result of hemopoietin-1 activity.
Pharmaceutical
Clinical trials on IL-1α have been carried out that are specifically designed to mimic the protective studies in animals. IL-1α has been administered to patients during receiving autologous bone marrow transplantation. The treatment with 50 ng/kg IL-1α from day zero of autologous bone marrow or stem cells transfer resulted in an earlier recovery of thrombocytopenia compared with historical controls. IL-1α is currently being evaluated in clinical trials as a potential therapeutic in oncology indications.
An anti-IL-1α therapeutic antibody, MABp1, is being tested in clinical trials for anti-neoplastic activity in solid tumors. Blocking the activity of IL-1α has the potential to treat skin diseases such as acne.
