Symbol Enterotoxin_a Pfam clan CL0084 SCOP 1lts | Pfam PF01375 InterPro IPR001144 SUPERFAMILY 1lts | |
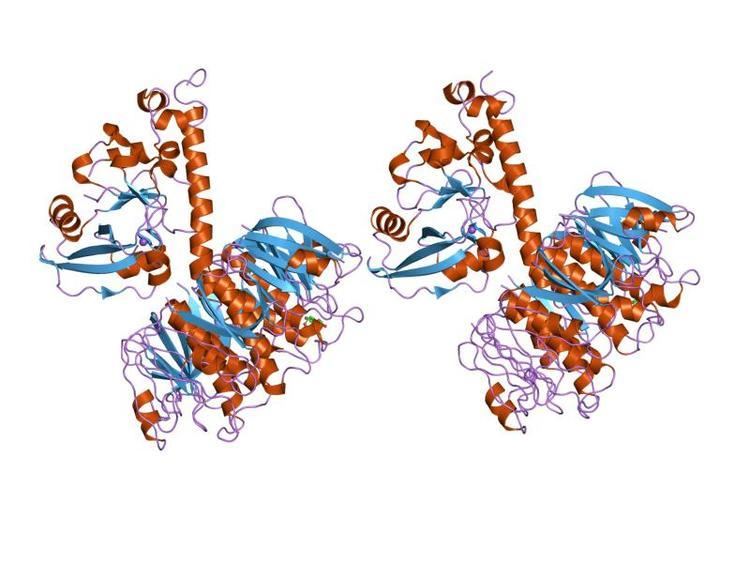 | ||
In molecular biology, the heat-labile enterotoxin family includes Escherichia coli heat-labile toxin and cholera toxin secreted by Vibrio cholerae. These toxins consist of an AB5 multimer structure, in which a pentamer of B chains has a membrane-binding function and an A chain is needed for enzymatic activity. The B subunits are arranged as a doughnut-shaped pentamer, each subunit participating in ~30 hydrogen bonds and 6 salt bridges with its two neighbours.
The A subunit has a less well-defined secondary structure. It predominantly interacts with the pentamer via the C-terminal A2 fragment, which runs through the charged central pore of the B subunits. A putative catalytic residue in the A1 fragment (Glu112) lies close to a hydrophobic region, which packs two loops together. It is thought that this region might be important for catalysis and membrane translocation.
The structural arrangement of type I and type II heat-labile enterotoxins are very similar, although they are antigenically distinct.
