 | ||
An HIV vaccine is a vaccine which would either protect individuals who do not have HIV from contracting that virus, or otherwise may have a therapeutic effect for persons who have or later contract HIV/AIDS. Currently, there is no effective HIV vaccine but many research projects managing clinical trials seek to create one. There is evidence that a vaccine may be possible. Work with monoclonal antibodies (MAb) has shown or proven that the human body can defend itself against HIV, and certain individuals remain asymptomatic for decades after HIV infection. Potential candidates for antibodies and early stage results from clinical trials have been announced.
Contents
- Overview
- Difficulties in developing an HIV vaccine
- HIV structure
- Animal model
- Clinical trials to date
- Phase I
- Phase II
- Phase III
- Economics of vaccine development
- Classification of all theoretically possible HIV vaccines
- Filtering virions from blood Phase I
- Approaches to catching the virion Phase I III VI VII
- Approaches to destroying or damaging the virion or its parts Phase I VII
- Blocking the replication Phase I
- Inhibiting process of phases drugs already used for this approach
- Inhibiting the functionality of infected cells Phase VI VII
- Future work
- Prophylactic drug
- References
One HIV vaccine candidate which showed some efficacy was studied in RV 144, which was a trial in Thailand beginning in 2003 and first reporting a positive result in 2009. Many trials have shown no efficacy, including the STEP study and HVTN 505 trials.
Overview
The urgency of the search for a vaccine against HIV stems from the AIDS-related death toll of over 25 million people since 1981. Indeed, in 2002, AIDS became the primary cause of mortality due to an infectious agent in Africa.
Alternative medical treatments to a vaccine do exist. Highly active antiretroviral therapy (HAART) has been highly beneficial to many HIV-infected individuals since its introduction in 1996 when the protease inhibitor-based HAART initially became available. HAART allows the stabilization of the patient’s symptoms and viremia, but they do not cure the patient of HIV, nor of the symptoms of AIDS. And, importantly, HAART does nothing to prevent the spread of HIV by people with undiagnosed infections. Introduction of safer sex measures to halt the spread of AIDS has proven difficult in the worst affected countries.
Therefore, an HIV vaccine is generally considered as the most likely, and perhaps the only way by which the AIDS pandemic can be halted. However, after over 30 years of research, HIV-1 remains a difficult target for a vaccine.
Difficulties in developing an HIV vaccine
In 1984, after the confirmation of the etiological agent of AIDS by scientists at the U.S. National Institutes of Health and the Pasteur Institute, the United States Health and Human Services Secretary Margaret Heckler declared that a vaccine would be available within two years.
However, the classical vaccination approaches that have been successful in the control of various viral diseases by priming the adaptive immunity to recognize the viral envelope proteins have failed in the case of HIV-1. Some have stated that an HIV vaccine may not be possible without significant theoretical advances.
There are a number of factors that cause development of an HIV vaccine to differ from the development of other classic vaccines:
HIV structure
The epitopes of the viral envelope are more variable than those of many other viruses. Furthermore, the functionally important epitopes of the gp120 protein are masked by glycosylation, trimerisation and receptor-induced conformational changes making it difficult to block with neutralising antibodies.
The ineffectiveness of previously developed vaccines primarily stems from two related factors.
The difficulties in stimulating a reliable antibody response has led to the attempts to develop a vaccine that stimulates a response by cytotoxic T-lymphocytes.
Another response to the challenge has been to create a single peptide that contains the least variable components of all the known HIV strains.
Animal model
The typical animal model for vaccine research is the monkey, often the macaque. Monkeys can be infected with SIV or the chimeric SHIV for research purposes. However, the well-proven route of trying to induce neutralizing antibodies by vaccination has stalled because of the great difficulty in stimulating antibodies that neutralise heterologous primary HIV isolates. Some vaccines based on the virus envelope have protected chimpanzees or macaques from homologous virus challenge, but in clinical trials, individuals who were immunised with similar constructs became infected after later exposure to HIV-1.
There are some differences between SIV and HIV that may introduce challenges in the use of an animal model.
As published on 27 November 2009 in Journal of Biology, there is a new animal model strongly resembling that of HIV in humans. Generalized immune activation as a direct result of activated CD4+ T cell killing - performed in mice allows new ways of testing HIV behaviour.
NIAID-funded SIV research has shown that challenging monkeys with a cytomegalovirus (CMV)-based SIV vaccine results in containment of virus. Typically, virus replication and dissemination occurs within days after infection, whereas vaccine-induced T cell activation and recruitment to sites of viral replication takes weeks. Researchers hypothesized that vaccines designed to maintain activated effector memory T cells might impair viral replication at its earliest stage.
Clinical trials to date
Several vaccine candidates are in varying phases of clinical trials.
Phase I
Most initial approaches have focused on the HIV envelope protein. At least thirteen different gp120 and gp160 envelope candidates have been evaluated, in the US predominantly through the AIDS Vaccine Evaluation Group. Most research focused on gp120 rather than gp41/gp160, as the latter are generally more difficult to produce and did not initially offer any clear advantage over gp120 forms. Overall, they have been safe and immunogenic in diverse populations, have induced neutralizing antibody in nearly 100% recipients, but rarely induced CD8+ cytotoxic T lymphocytes (CTL). Mammalian derived envelope preparations have been better inducers of neutralizing antibody than candidates produced in yeast and bacteria. Although the vaccination process involved many repeated "booster" injections, it was very difficult to induce and maintain the high anti-gp120 antibody titers necessary to have any hope of neutralizing an HIV exposure.
The availability of several recombinant canarypox vectors has provided interesting results that may prove to be generalizable to other viral vectors. Increasing the complexity of the canarypox vectors by inclusion of more genes/epitopes has increased the percent of volunteers that have detectable CTL to a greater extent than did increasing the dose of the viral vector. Importantly, CTLs from volunteers were able to kill peripheral blood mononuclear cells infected with primary isolates of HIV, suggesting that induced CTLs could have biological significance. In addition, cells from at least some volunteers were able to kill cells infected with HIV from other clades, though the pattern of recognition was not uniform among volunteers. Canarypox is the first candidate HIV vaccine that has induced cross-clade functional CTL responses. The first phase I trial of the candidate vaccine in Africa was launched early in 1999 with Ugandan volunteers. The study determined the extent to which Ugandan volunteers have CTL that are active against the subtypes of HIV prevalent in Uganda, A and D.
Other strategies that have progressed to phase I trials in uninfected persons include peptides, lipopeptides, DNA, an attenuated Salmonella vector, p24, etc. Specifically, candidate vaccines that induce one or more of the following are being sought:
In 2011, researchers in National Biotech Centre in Madrid unveiled data from the Phase I clinical trial of their new vaccine, MVA-B. The vaccine was effective in inducing an immunological response in 92% of the healthy subjects.
Phase II
On December 13, 2004, the HIV Vaccine Trials Network (HVTN) began recruiting for the STEP study, a 3,000-participant phase II clinical trial of a novel HIV vaccine, at sites in North America, South America, the Caribbean and Australia. The trial was co-funded by the National Institute of Allergy and Infectious Diseases (NIAID), which is a division of the National Institutes of Health (NIH), and the pharmaceutical company Merck & Co. Merck developed the experimental vaccine called V520 to stimulate HIV-specific cellular immunity, which prompts the body to produce T cells that kill HIV-infected cells. In previous smaller trials, this vaccine was found to be safe, because of the lack of adverse effects on the patients. The vaccine showed induced cellular immune responses against HIV in more than half of volunteers.
V520 contains a weakened adenovirus that serves as a carrier for three subtype B HIV genes (gag / pol / nef). Subtype B is the most prevalent HIV subtype in the regions of the study sites. Adenoviruses are among the main causes of upper respiratory tract ailments such as the common cold. Because the vaccine contains only three HIV genes housed in a weakened adenovirus, study participants cannot become infected with HIV or get a respiratory infection from the vaccine. It was announced in September 2007 that the trial for V520 would be discontinued after it determined that the vaccination appeared associated with an increased risk of HIV infection in some recipients. The foremost issue facing the rAd5 adenovirus that was used is the high prevalence of the adenovirus-specific antibodies as a result of prior exposure to the virus. Adenovirus vectors and many other viral vectors currently used in HIV vaccines, will induce a rapid memory immune response against the vector. This results in an impediment to the development of a T cell response against the inserted antigen (HIV antigens) Additionally, it appears that V520 may have made some recipients more receptive to infection by HIV-1.
The HVTN expected to finish the study in 2009, but ceased further treatment administration and declared the vaccine ineffective at preventing HIV-infection in September 2007. The results of the trial have caused some to call for a reexamination of vaccine development strategies.
Biosantech has developed a novel vaccine called Tat Oyi, which aims at the tat protein. It is reported that the company’s HIV vaccine candidate is not toxic to 48 HIV-positive patients enrolled in a double-blind study taking place in France. If this experimental vaccine is proved to be safe and effective, people with HIV could receive several injections to control their virus permanently instead of taking antiretroviral treatment. Corinne Treger, the chief executive of French pharmaceutical company Biosantech, claims that if a new trial in 80 patients in 2015 goes well, she said the vaccine could be available in 2017. Results of phase 2 trials of 200 enrollees published in June 2016 were shown to be effective in increasing CD4 cell levels.
Phase III
In February 2003, VaxGen announced that their AIDSVAX vaccine was a failure in North America as there was not a statistically significant reduction of HIV infection within the study population. AIDSVAX was also a component of the prime boost (ALVAC/AIDSVAX) RV 144 vaccine study in Thailand that showed marginal successful results. In both cases the vaccines targeted gp120 and were specific for the geographical regions. The Thai trial was the largest AIDS vaccine trial to date when it started.
In October 2009, the results of the RV 144 trial were published. Initial results, released in September 2009 prior to publication of complete results, were encouraging for scientists in search of a vaccine. The study involved 16,395 participants who did not have HIV infection, 8197 of whom were given treatment consisting of two experimental vaccines targeting HIV types B and E that are prevalent in Thailand, while 8198 were given a placebo. The participants were tested for HIV every six months for three years. After three years, the vaccine group saw HIV infection rates reduced by more than 30% compared with those in the placebo group. However, after taking into account the seven people who had HIV infections at the time of their vaccination (two in the placebo group, five in the vaccine group) the percentage dropped to 26%.
Further analysis presented at a 2011 AIDS conference in Bangkok revealed that participants receiving vaccines in the RV 144 trial who produced IgG antibodies against the V2 loop of the HIV outer envelope were 43% less likely to become infected than those who did not, while IgA production was associated with a 54% greater risk of infection than those who did not produce the antibodies (but not worse than placebo). Viruses collected from vaccinated participants possessed mutations in the V2 region. Tests of a vaccine for SIV in monkeys found greater resistance to SIV in animals producing antibodies against this region. For these reasons further vaccine development was expected to focus heavily on vaccines designed to provoke an IgG reaction against the V2 loop.
In 2016, the trial HVTN 702 was launched in South Africa. The trial uses the ALVAC-HIV vaccine and a two-component gp120 protein subunit vaccine with an adjuvant. The trial aims to enroll 5,400 men and women in order to test whether the regimen is safe, tolerable and effective at preventing HIV infection.
Economics of vaccine development
A July 2012 report of the HIV Vaccines & Microbicides Resource Tracking Working Group estimates that $845 million was spent on AIDS vaccine research in 2011.
Economic issues with developing an AIDS vaccine include the need for advance purchase commitment (or advance market commitments) because after an AIDS vaccine has been developed, governments and NGOs may be able to bid the price down to marginal cost.
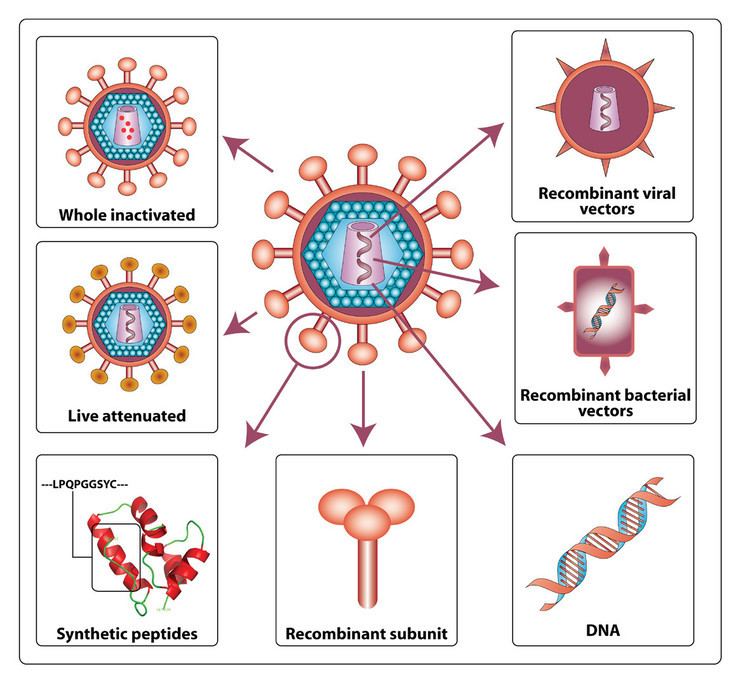
Classification of all theoretically possible HIV vaccines
Theoretically, any possible HIV vaccine must inhibit or stop the HIV virion replication cycle. The targets of the vaccine are the following phases of the HIV virion cycle:
Therefore, the following list comprises the current possible approaches for the HIV vaccine
Filtering virions from blood (Phase I)
Approaches to catching the virion (Phase I-III, VI, VII)
Approaches to destroying or damaging the virion or its parts (Phase I-VII)
Here, “damage” means inhibiting or stopping the ability of virion to process any of the Phase II-VII. Here are the different classification of methods:
Blocking the replication (Phase I)
Inhibiting process of phases (drugs already used for this approach)
Inhibiting the functionality of infected cells (Phase VI- VII)
Inhibiting the life functions of infected cells:
Future work
According to Gary J. Nabel of the Vaccine Research Center, NIH, in Bethesda, Maryland, several hurdles must be overcome before scientific research will culminate in a definitive AIDS vaccine. First, greater translation between animal models and human trials must be established. Second, new, more effective, and more easily produced vectors must be identified. Finally, and most importantly, there must arise a robust understanding of the immune response to potential vaccine candidates. Emerging technologies that enable the identification of T-cell-receptor specificities and cytokine profiles will prove valuable in hastening this process. In July 2012 a science group speculated that an effective vaccine for HIV would be completed in 2019.
A killed whole HIV vaccine, SAV001, that has had success in the US FDA phase 1 human clinical trial in Sep. 2013. This HIV vaccine uses a "dead" version of HIV-1 for the first time. The outcome of the phase 1 human clinical trial has turned out that the vaccine has shown no serious adverse effects while boosting HIV-1 specific antibody. According to Dr. Chil-Yong Kang of Western University's Schulich School of Medicine & Dentistry in Canada, the developer of this vaccine, the antibody against gp120 surface antigen and p24 capsid anigen increased to 8-fold and 64-fold, respectively after vaccination.
There have been reports that HIV patients coinfected with GBV-C can survive longer than those without GBV-C, but the patients may be different in other ways. There is current active research into the virus' effects on the immune system in patients coinfected with GBV-C and HIV.
A promising new approach to a live attenuated HIV-1 vaccine is being pursued by scientists, using a genetically modified form of the HIV virus. The new method involves manipulating the virus' codons, this is a sequence of three nucleotides that form genetic code, to rely on an unnatural amino acid for proper protein translation, which allows it to replicate. Because this amino acid is foreign to the human body, the virus cannot continue to reproduce.
Prophylactic drug
On July 16, 2012, The Food and Drug Administration approved the first drug shown to reduce the risk of HIV infection. The agency approved Gilead Sciences' pill Truvada (Emtricitabine and Tenofovir) as a preventive measure for people who are at high risk of getting HIV through sexual activity. The drug is meant to be used with safe sex practices including the use of condoms. This prophylactic drug is also used in combination with other mediums to treat HIV positive children over the age of twelve years old and HIV positive adults. Doctors are required to test patients for HIV before prescribing Truvada every three months. Along with that professionals are also required to screen their patients who have contracted HIV while on the drug to track for resistance of the prophylactic.
