 | ||
Gliflozin drugs are a class of medications that inhibit reabsorption of glucose in the kidney and therefore lower blood sugar. They act by inhibiting sodium-glucose transport protein 2 (SGLT2), and are therefore also called SGLT2 inhibitors. Gliflozins are used in the treatment of type II diabetes mellitus (T2DM). As studied on canagliflozin, a member of this class, gliflozins enhance blood sugar control as well as reduce body weight and systolic and diastolic blood pressure.
Contents
Medical uses
The gliflozins are used to treat type 2 diabetes mellitus.
Adverse effects
In May 2015, the United States' Food and Drug Administration issued a warning that the gliflozins canagliflozin, dapagliflozin, and empagliflozin may lead to ketoacidosis.
Other side effects of gliflozins include increased risk of urinary tract infections, candidal vulvovaginitis and low blood sugar (hypoglycemia).
Drug-Drug Interaction
Interactions are very important for SGLT2 inhibitors because most T2DM patients are taking many other medications. There is low risk of clinically relevant pharmacokinetics drug interactions because of the metabolic pathway of SGLT2. According to Bhartia and his co-workers, it is safe to consume dapagliflozin along with pioglitazone, metformin, glimepiride, or sitagliptin and dose adjustment is unnecessary for either drug. It is unlikely that food intake has clinical meaningful impact on the efficacy of dapagliflozin, therefore it can be administered without regard to meals.
Members
Several drug candidates have been developed or are currently undergoing clinical trials, including:
Mechanism of action
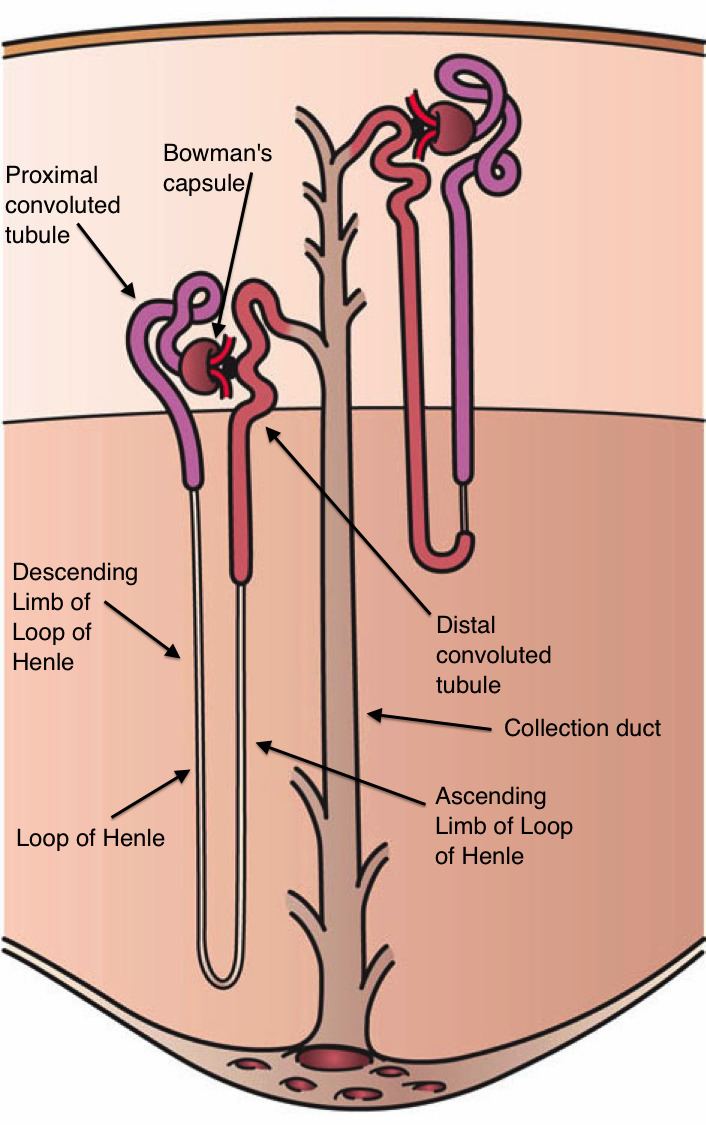
SGLTs are responsible for mediating glucose reabsorption in the kidneys, as well as in the gut and the heart. SGLT-2 is primarily expressed in the kidney on the epithelial cells lining the first segment of the proximal convoluted tubule. It is the major transport protein that promotes reabsorption from the glomerular filtration glucose back into circulation and is responsible for approximately 90% of the kidney's glucose reabsorption. By inhibiting SGLT-2, medications of the gliflozin class prevent the kidneys' reuptake of glucose from the glomerular filtrate and subsequently lower the glucose level in the blood and promote the excretion of glucose in the urine (glucosuria).
Dapagliflozin is an example of an SGLT-2 inhibitor, it is a competitive, highly selective inhibitor of SGLT. It acts via selective and potent inhibition of SGLT-2, and its activity is based on each patient’s underlying blood sugar control and kidney function. The results are decreased kidney reabsorption of glucose, glucosuria effect increases with higher level of glucose in the blood circulation. Therefore, dapagliflozin reduces the blood glucose concentration with a mechanism that is independent of insulin secretion and sensitivity, unlike many other antidiabetic drugs. Functional pancreatic β-cells are not necessary for the activity of the drug so it is convenient for patients with diminished β-cell function.
Sodium and glucose are co-transported by the SGLT-2 protein into the tubular epithelial cells across the brush-border membrane of the proximal convoluted tubule. This happens because of the sodium gradient between the tubule and the cell and therefore provides a secondary active transport of glucose. Glucose is later reabsorbed by passive transfer of endothelial cells into the interstitial glucose transporter protein.
The use of dapagliflozin with other oral antidiabetic agents act synergistically with virtually no increased risk of developing low blood sugar levels.
Pharmacology
The elimination half-life, bioavailability, protein binding, and other pharmacokinetic parameters of various drugs of this class are present in table 1. These drugs are excreted in the urine as inactive metabolites.
TABLE 1: PHARMACOKINETIC PARAMETERS OF VARIOUS SGLT-2 INHIBITORSIn studies that were made on healthy people and people with type II diabetes mellitus, who were given dapagliflozin in either single ascending dose (SAD) or multiple ascending dose (MAD) showed results that confirmed a pharmacokinetic profile of the drug. With dose-dependent concentrations the half-life is about 12–13 hours, Tmax 1–2 hours and it is protein-bound, so the medication has a rapid absorption and minimal excretion by the kidney.
Dapagliflozin disposition is not evidently affected by BMI or body weight, therefore the pharmacokinetic findings are expected to be applicable to patients with a higher BMI. Dapagliflozin resulted in dose-dependent increases excretions in urinary glucose, up to 47g/d following single-dose administration, which can be expected from its mechanism of action, dapagliflozin.
In some long term clinical studies that have been made on dapagliflozin, dapagliflozin was associated with a decrease in body weight which was statistically superior compared to placebo or other active comparators. It is primarily associated with caloric rather than fluid loss.
History
Phlorizin is a molecule with SGLT inhibiting properties, and served an important role in the development of the gliflozin class of drugs.
