Entrez 373156 | Ensembl ENSG00000197448 | |
 | ||
Aliases GSTK1, GST, GST 13-13, GST13, GST13-13, GSTK1-1, hglutathione S-transferase kappa 1 External IDs MGI: 1923513 HomoloGene: 41075 GeneCards: GSTK1 | ||
Glutathione S-transferase kappa 1 (GSTK1) is an enzyme that in humans is encoded by the GSTK1 gene which is located on chromosome seven. It belongs to the superfamily of enzymes known as glutathione S-transferase (GST), which are mainly known for cellular detoxification. The GSTK1 gene consists of eight exons and seven introns and although it is a member of the GST family, its structure has been found to be similar to bacterial HCCA (2-hydroxychromene-2-carboxylate) isomerases and bacterial disulphide-bond-forming DsbA oxidoreductase. This similarity has later allowed the enzyme GSTK1 to be renamed to DsbA-L. Research has also suggested that several variations of the GSTK1 gene can be responsible for metabolic diseases and certain types of cancer.
Contents
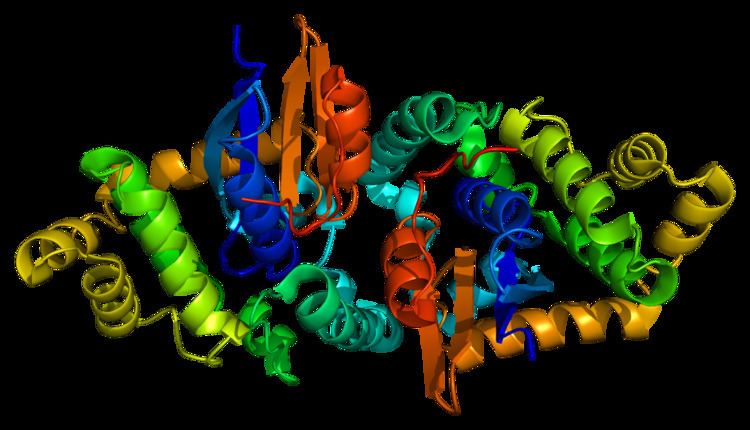
Structure
The GSTK1 enzyme is a homodimer and, like all GSTs, it contains a TRX-like domain and a helical domain. However, the GSTK1 is substantially different in its secondary structure compared to the other GSTs. The helical domain has been observed to be placed between the βαβ and ββα motifs of the TRX-like domain, rather than the TRX-like domain and the C-terminal helical domain being connected together by a short linker of alpha-helixes as normally seen in GSTs. Also, the GSTK1 dimer employs a butterfly shape and not a V-shaped crevice like in the other classes. As for the GSTK1 gene, it is ~5 kb long, has eight exons, is located on chromosome 7q34, and includes an initiator element at the transcription start site instead of a TATA or a CCAAT box.
Function
GSTK1 has been observed in promoting adiponectin multimerization in the endoplasmic reticulum (ER). How the GSTK1 is able to do this is still unknown. The enzyme can also prevent ER stress and ER stress induced adiponectin down-regulation, which implies that GSTK1 assists the ER’s functions. GSTK1 is not only located in the ER, but also in the mitochondria of hepatocytes. This indicates that GSTK1 could be vital to the ER, the mitochondria, and the interactions between the two organelles; however, there is still limited knowledge about this and more studies must be conducted to find out.
The discovery of GSTK1 in the peroxisome of a cell has further led to more studies based on its function. It has been suggested that, based on the GSTA enzyme, GSTK1 could play a role in the buffering system of acyl-CoA and xenobiotic-CoA and be involved in their binding activities. Also, it is hypothesized that GSTK1 is responsible for the detoxification of lipid peroxides, which are created in the peroxisome. This is based on the fact that there is peroxidase activity towards three substrates: tert-butyl hydroperoxide, cumene hydroperoxide, and 15-S-hydroperoxy-5,8,11,13-eicosatetraenoic acid.
Clinical significance
The amount of expression of adiponectin has been observed to be related to diseases such as insulin resistance, obesity, and type 2 diabetes. Decreased amounts of the protein indicates that there is a higher probability of receiving said diseases. Because the GSTK1 is seen to play a role in the multimerization of adiponectin, this enzyme can regulate the concentration of adiponectin and thus enhance insulin sensitivity and protect against diabetes. Also, the GSTK1 gene is unregulated when it is inflicted with oxidative stress and are over expressed in many tumors leading to difficulties during cancer chemotherapy. Moreover, GSTK1 gene expression has been seen to increase significantly in correlation to drug resistance in tumor cells such as erythroleukemia and mammary adenocarcinoma suggesting that it, along with GSTP1 and GSTA4, could be responsible for the drug resistance.
GSTK1 can also be a potential tool to help investigate cancer. Tyrosine phosphorylated proteins are responsible for many of the cell functions such as the cell’s growth, division, adhesion, and motility. These activities are also very related to cancer and thus studying this protein could allow access to information which could classify tumors for prognosis and prediction. Due to GSTK1’s C-terminal SH2 domain, tyrosine phosphorylated proteins can bind to it and allow for easier detection to which the protein can be studied.
Interactions
GSTK1 has been seen to interact with:
