 | ||
Flavan-3-ols (sometimes referred to as flavanols) are derivatives of flavans that use the 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. These compounds include catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins.
Contents
- Catechin and the gallates
- Biosynthesis of epicatechin
- Potential health effects of catechins
- Possible reduced benefits
- Analysis
- Other uses
- References
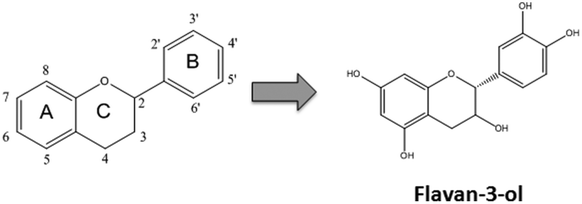
Flavanols (with an "a") are not to be confused with flavonols (with an "o"), a class of flavonoids containing a ketone group.

The single-molecule (monomer) catechin, or isomer epicatechin (see diagram), adds four hydroxyls to flavan-3-ol, making building blocks for concatenated polymers (proanthocyanidins) and higher order polymers (anthocyanidins).
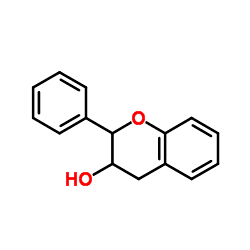
Flavanols possess two chiral carbons, meaning four diastereoisomers occur for each of them.
Catechins are distinguished from the yellow, ketone-containing flavonoids such as quercitin and rutin, which are called flavonols. Early use of the term bioflavonoid was imprecisely applied to include the flavanols, which are distinguished by absence of ketone(s). Catechin monomers, dimers, and trimers (oligomers) are colorless. Higher order polymers, anthocyanidins, exhibit deepening reds and become tannins.
Catechin and the gallates
Catechin and epicatechin are epimers, with (-)-epicatechin and (+)-catechin being the most common optical isomers found in nature. Catechin was first isolated from the plant extract catechu, from which it derives its name. Heating catechin past its point of decomposition releases pyrocatechol (also called catechol), which explains the common origin of the names of these compounds.
Epigallocatechin and gallocatechin contain an additional phenolic hydroxyl group when compared to epicatechin and catechin, respectively, similar to the difference in pyrogallol compared to pyrocatechol.
Catechin gallates are gallic acid esters of the catechins; an example is epigallocatechin gallate, which is commonly the most abundant catechin in tea.
Biosynthesis of (-)-epicatechin
The flavonoids are products from a cinnamoyl-CoA starter unit, with chain extension using three molecules of malonyl-CoA. Reactions are catalyzed by a type III PKS enzyme. These enzyme do not use ACPSs, but instead employ coenzyme A esters and have a single active site to perform the necessary series of reactions, e.g. chain extension, condensation, and cyclization. Chain extension of 4-hydroxycinnamoyl-CoA with three molecules of malonyl-CoA gives initially a polyketide (Figure 1), which can be folded. These allow Claisen-like reactions to occur, generating aromatic rings.
Figure 1:Schematic overview of the flavan-3-ol (-)-epicatechin biosynthesis in plants: Enzymes are indicated in blue, abbreviated as follows: E1, phenylalanine ammonia lyase (PAL), E2, tyrosine ammonia lyase (TAL), E3, cinnamate 4-hydroxylase, E4, 4-coumaroyl: CoA-ligase, E5, chalcone synthase (naringenin-chalcone synthase), E6, chalcone isomerase, E7, Flavonoid 3'-hydroxylase, E8, flavonone 3-hydroxylase, E9, dihydroflavanol 4-reductase, E10, anthocyanidin synthase (leucoanthocyanidin dioxygenase), E11, anthocyanidin reductase. HSCoA, Coenzyme A. L-Tyr, L-tyrosine, L-Phe, L-phenylalanine.
Potential health effects of catechins
The supposed health benefits of catechins have been studied extensively in humans and animal models, but there are no proven effects that apply to human health. Until 2013, neither the Food and Drug Administration nor the European Food Safety Authority had approved any health claim for catechins or approved any as pharmaceutical drugs. Moreover, several companies have been cautioned by the FDA over misleading health claims.
In 2014, the European Food Safety Authority approved the following health claim for cocoa products containing 200 mg of flavanols and meeting the qualification in dietary supplement products: “cocoa flavanols help maintain the elasticity of blood vessels, which contributes to normal blood flow”.
Possible reduced benefits
An editorial warned against increasing one’s intake of dark chocolate to improve health because the beneficial compounds, suggested to be flavanols, are sometimes removed due to their bitter taste without an indication on the label. Additionally, such a product is high in fat, sugar and Calories, contributing to a poor diet if consumed in large amounts.
Analysis
Fluorescence-lifetime imaging microscopy (FLIM) can be used to detect flavanols in plant cells
Other uses
Recent study tested catechins employed to coat nanoparticles of iron oxides in the blood. These particles allow visualization of vessels - and especially cancer tumors in mice - in an MRI exam. The nanoparticles would clump together without the catechin coating.
