 | ||
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.
Yield can be broken down by:
- Individual isotope
- Chemical element spanning several isotopes of different mass number but same atomic number.
- Nuclei of a given mass number regardless of atomic number. Known as "chain yield" because it represents a decay chain of beta decay.
Isotope and element yields will change as the fission products undergo beta decay, while chain yields do not change after completion of neutron emission by a few neutron-rich initial fission products (delayed neutrons), with halflife measured in seconds.
A few isotopes can be produced directly by fission, but not by beta decay because the would-be precursor with atomic number one greater is stable and does not decay. Chain yields do not account for these "shadowed" isotopes; however, they have very low yields (less than a millionth as much as common fission products) because they are far less neutron-rich than the original heavy nuclei.
Yield is usually stated as percentage per fission, so that the total yield percentages sum to 200%. Less often, it is stated as percentage of all fission products, so that the percentages sum to 100%. Ternary fission, about 0.2% to 0.4% of fissions, also produces a third light nucleus such as helium-4 (90%) or tritium (7%).
Mass vs. yield curve
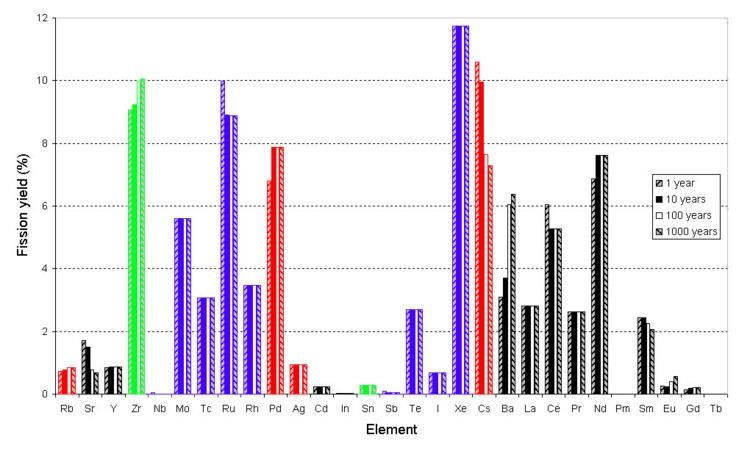
If a graph of the mass or mole yield of fission products against the atomic number of the fragments is drawn then it has two peaks, one in the area zirconium through to palladium and one at xenon through to neodymium. This is because the fission event causes the nucleus to split in an asymmetric manner, as nuclei closer to magic numbers are more stable. Yield vs. Z - This is a typical distribution for the fission of uranium. Note that in the calculations used to make this graph the activation of fission products was ignored and the fission was assumed to occur in a single moment rather than a length of time. In this bar chart results are shown for different cooling times (time after fission).
Because of the stability of nuclei with even numbers of protons and/or neutrons the curve of yield against element is not a smooth curve. It tends to alternate.
In general, the higher the energy of the state that undergoes nuclear fission, the more likely a symmetric fission is, hence as the neutron energy increases and/or the energy of the fissile atom increases, the valley between the two peaks becomes more shallow; for instance, the curve of yield against mass for Pu-239 has a more shallow valley than that observed for U-235, when the neutrons are thermal neutrons. The curves for the fission of the later actinides tend to make even more shallow valleys. In extreme cases such as 259Fm, only one peak is seen.
Yield is usually expressed relative to number of fissioning nuclei, not the number of fission product nuclei, that is, yields should sum to 200%.
The table in the next section gives yields for notable radioactive (with halflife greater than one year, plus iodine-131) fission products, and (the few most absorptive) neutron poison fission products, from thermal neutron fission of U-235 (typical of nuclear power reactors), computed from [1].
The yields in the table sum to only 45.5522%, including 34.8401% which have halflife greater than one year:
The remainder and the unlisted 54.4478% decay with halflife less than one year into nonradioactive nuclei.
This is before accounting for the effects of any subsequent neutron capture, e.g.:
Besides fission products, the other types of radioactive products are
