ATC code G03DC04 (WHO) PubChem CID 5284557 Molar mass 312.446 g/mol | CAS Number 434-03-7 ChemSpider 4447612 CAS ID 434-03-7 | |
 | ||
Trade names Etherone, Ethisteron, Luteosterone, Lutocyclin, Lutocylol, Pranone, Progesteron lingvalete, Progestoral, Proluton C, Syngestrotabs, Trosinone Synonyms Etisteron, Pregnin, Ethindrone, Ethinyltestosterone, Ethynyltestosterone | ||
Ethisterone (INN, USAN, BAN) (brand names Pranone, Progestoral, Lutocylol, Proluton C, many others), also known as 17α-ethinyltestosterone, pregneninolone, or anhydrohydroxyprogesterone, is a steroidal progestin with androgenic activity which is derived from testosterone and was introduced for medical use in 1939. It was the second progestogen to be marketed (intramuscular progesterone was introduced as Proluton in 1934) and was both the first orally active progestogen and the first progestin (or synthetic progestogen) to be introduced. Although ethisterone has largely been superseded by newer drugs and is now little used, it continues to be available in some countries. Moreover, the 19-nortestosterone progestins, such as norethisterone, are derived from ethisterone and are widely used as hormonal contraceptives and for other indications.
Contents

Pharmacology
Ethisterone is described as a relatively weak progestogen, similarly to its analogue dimethisterone.
Androgenic activity
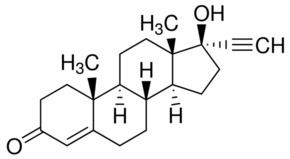
Based on in vitro research, ethisterone and norethisterone are about equipotent in their EC50 values for the androgen receptor (AR), whereas, conversely, norethisterone shows markedly increased potency relative to ethisterone in terms of its EC50 for the progesterone receptor (PR). As such, there is a considerable separation in the ratios of androgenic and progestogenic activity for ethisterone and norethisterone. Moreover, at the larger dosages in which it is used to achieve equivalent progestogenic effect, ethisterone has strong androgenic effects relative to norethisterone and other 19-nortestosterone progestins, and this has limited its clinical use.

Due to its androgenic activity, ethisterone has been associated with the masculinization of female fetuses in women who have taken it during pregnancy.
Estrogenic activity
High dosages of norethisterone and noretynodrel, which are 19-nortestosterone derivatives of ethisterone, were found to be associated with high rates of estrogenic side effects such as breast enlargement in women and gynecomastia in men, as well as with improvement of menopausal symptoms in postmenopausal women. In contrast, ethisterone and other progestogens such as progesterone and hydroxyprogesterone caproate were not associated with such effects, suggesting that they are either not estrogenic or are only weak so. As such, ethisterone does not appear to share the estrogenic activity of norethisterone and noretynodrel.
Chemistry
Ethisterone is also known by the following synonyms:
Closely related analogues of ethisterone include vinyltestosterone, allyltestosterone, methyltestosterone, ethyltestosterone, and propyltestosterone.
History
Ethisterone was synthesized in 1938 by Hans Herloff Inhoffen, Willy Logemann, Walter Hohlweg, and Arthur Serini at Schering AG in Berlin. It was derived from testosterone via ethynylation at the C17α position, and it was hoped, that, analogously to estradiol and ethinylestradiol, ethisterone would be an orally active form of testosterone. However, the androgenic activity of ethisterone was attenuated and it showed considerable progestogenic activity. As such, it was developed as a progestogen instead and was marketed in Germany in 1939 as Proluton C and by Schering in the U.S. in 1945 as Pranone, among other brand names.
