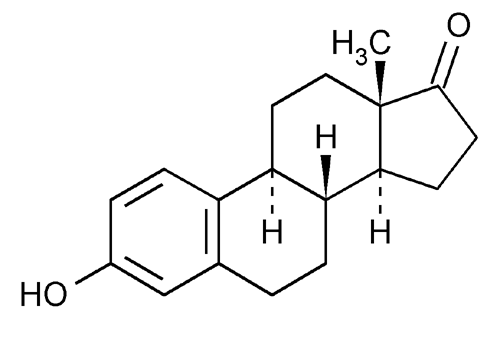
Legal status US: ℞-only Biological half-life 19 hours PubChem CID 5870 Molar mass 270.366 g/mol Classification Estrogen | Protein binding >95% CAS Number 53-16-7 Formula C18H22O2 CAS ID 53-16-7 | |
 | ||
ATC code G03CA07 (WHO) G03CC04 (WHO) | ||
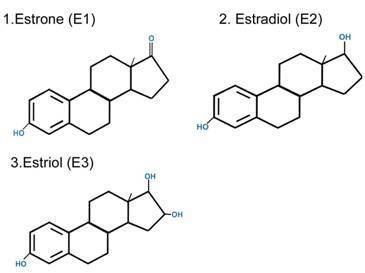
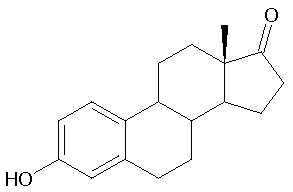
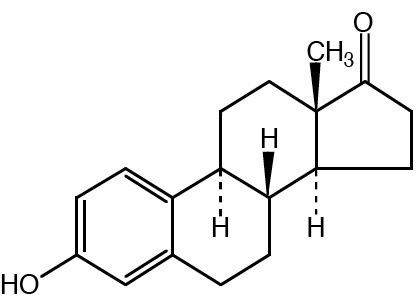
Estrone (American English) (abbreviated as E1), or oestrone (British English), also known as estra-1,3,5(10)-trien-3-ol-17-one, is an estrogenic hormone secreted by the ovary as well as adipose tissue. It is one of several natural estrogens, which also include estriol and estradiol. Estrone is the least abundant of the three hormones; estradiol is present almost always in the reproductive female body, and estriol is abundant primarily during pregnancy.
Contents
- Estrone meaning
- Biosynthesis
- Chemistry
- Environmental exposure
- Women
- Men
- Eyes
- Skin
- Inhalation
- Ingestion
- References
Estrone was discovered and isolated by German chemist Adolf Butenandt. It was formerly marketed under the brand name Theelin in the United States for intramuscular injection, but this formulation is no longer available.
Estrone meaning
Biosynthesis

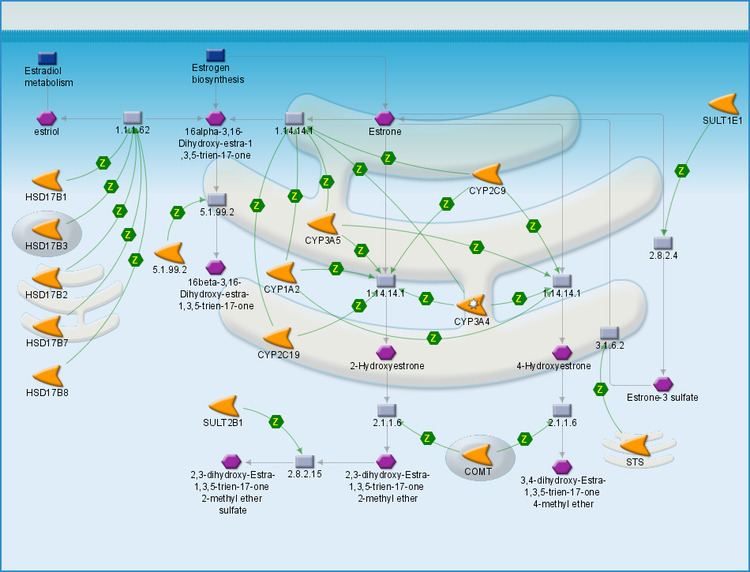
Estrone is synthesized via aromatase from 4-androstenedione. The conversion consists of the demethylation of the C19 position and the aromatization of the A ring. This reaction is similar to the conversion of testosterone to estradiol via aromatase. Estrone is also reversibly produced from estradiol via 17β-hydroxysteroid dehydrogenase.
Chemistry
Estrone has the chemical name 3-hydroxyestra-1,3,5(10)-triene-17-one or estra-1,3,5(10)-triene-3-ol-17-one and the chemical formula C18H22O2. It is an odorless, solid crystalline powder, white in color with a melting point of 254.5 °C (490 °F) and a specific gravity of 1.23. At high temperatures estrone is combustible and the products of combusting estrone are carbon monoxide (CO) and carbon dioxide (CO2).

Estrone is also named aquacrine, bestrone, crinovaryl, folliculin, 1,3,5-estratriene-3-ol-17-one, estro-a, estronol, erystogen, 3-hydroxyestra-1,3,5(10)-trien-17-one, kestrone 5, propagon-s, menagen, theelin, theogen, destrone, crystogen, oestrin, 1,3,5(10)-estratrien-3-ol-17-one, 1,3,5(10)-oestratrien-3-ol-17-one, and oestrone.
Environmental exposure
Estrone is known to be a carcinogen for human females as well as a cause of breast tenderness or pain, nausea, headache, hypertension, and leg cramps in the context of non-endogenous exposure. In men, estrone has been known to cause anorexia, nausea, vomiting, and erectile dysfunction. Estrone is relevant to health and disease states because of its conversion to estrone sulfate, a long-lived derivative. Estrone sulfate acts as a reservoir that can be converted as needed to the more active estradiol. It is the predominant estrogen in postmenopausal women.

The use of estrone in norsteroid, a derivative of steroids, and in medications can cause its release into the environment through waste streams.
Women
Estrone has been proven to be a known carcinogen for human females. The Occupational Safety and Health Administration (OSHA) classifies estrone as an OSHA Select carcinogen. Exposure to estrone can cause breast tenderness or pain, cervical hyper secretion, menstrual disorders including menorrhagia and metrorrhagia, nausea, headache, hypertension, leg cramps, vision disturbances, and endometriosis pain in women. Mothers lactating can also experience a decrease in the production of breast milk. Estrone can be found in the urine of pregnant women and can also be excreted in feces. Estrone is commonly produced in large quantities of the livers of transgender women who take oral estradiol. This is a result of first pass metabolism converting estradiol to estrone.
Men
Exposure to estrone can cause anorexia, nausea, vomiting, edema, feminization including gynecomastia and erectile dysfunction in men.
Eyes

If exposure of estrone to the eyes occurs, contacts should be removed and rinsed with water for 15 minutes. If any irritation occurs medical attention may be necessary. Exposure of estrone to the eyes can be prevented through the use of safety goggles.
Skin
If exposure of estrone to the skin occurs, the effected area should be washed with soap and water and then covered with an emollient or moisturizer. Medical attention may be necessary if irritation persists. Exposure of estrone can be prevented through the use protective gloves, the wearing of pants, shirts that cover the entire upper body, and lab coat.
Inhalation
If inhalation of estrone occurs, one should move into the fresh air. If breathing ceases, artificial respiration should be administered. For difficult breathing, oxygen should be given. Medical attention may be necessary if breathing continues to be difficult. Inhalation of estrone can be prevented through the use of a dust respirator.
Ingestion
If estrone is ingested, vomiting should not be induced. Medical attention may be necessary.
