 | ||
The effective nuclear charge (often symbolized as
Contents
Calculation
In an atom with one electron, that electron experiences the full charge of the positive nucleus. In this case, the effective nuclear charge can be calculated from Coulomb's law.
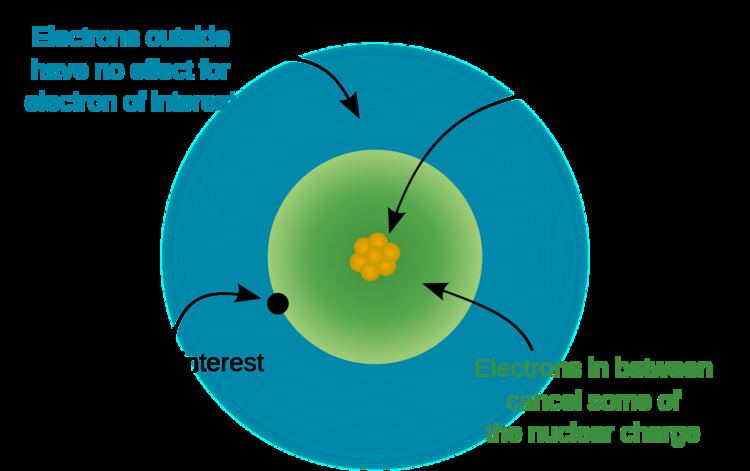
However, in an atom with many electrons, the outer electrons are simultaneously attracted to the positive nucleus and repelled by the negatively charged electrons. The effective nuclear charge on such an electron is given by the following equation:
where
Z is the number of protons in the nucleus (atomic number), andS is the average number of electrons between the nucleus and the electron in question (the number of non-valence electrons).S can be found by the systematic application of various rule sets, the simplest of which is known as "Slater's rules" (named after John C. Slater). Douglas Hartree defined the effective Z of a Hartree–Fock orbital to be:
where
Values
Updated values of screening constants were provided by Clementi et al.
