Type Whole antibody Target IL4 receptor alpha CAS Number 1190264-60-8 | ATC code none ChemSpider none | |
 | ||
Tsw 1 year successful new dupilumab treatment
Dupilumab is a monoclonal antibody designed for the treatment of atopic diseases. This drug was developed by Regeneron Pharmaceuticals. The US Food and Drug Administration has designated dupilumab a “breakthrough therapy” which is designed to speed promising new drugs to market.
Contents
- Tsw 1 year successful new dupilumab treatment
- Dupilumab potentially revolutionize the treatment of atopic dermatitis
- Mechanism of action
- Medical uses
- Clinical trials
- References
Dupilumab potentially revolutionize the treatment of atopic dermatitis
Mechanism of action
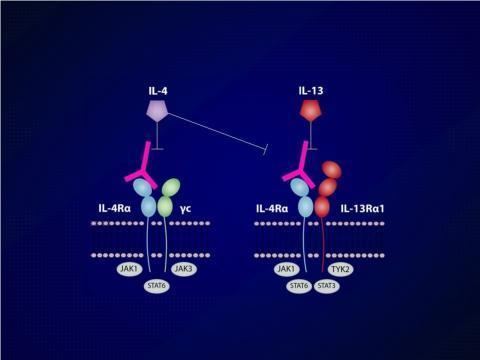
It binds to the alpha subunit of the interleukin-4 receptor (IL-4Rα). Through blockade of IL-4Rα, dupilumab modulates signaling of both the interleukin 4 and interleukin 13 pathway.
Medical uses
The interleukin 4 and 13 pathways have been implicated in the pathophysiology of allergic disease, particularly asthma and atopic dermatitis.
Clinical trials
In 2013 mid-stage data was presented at the American Thoracic Society meeting and published in the NEJM demonstrating an 87% (placebo: 67%) reduction in asthma exacerbations in patients with moderate-to-severe allergic asthma. In an atopic dermatitis study, 85%(36 percent or 85 out of 671 patients) of patients improved their symptoms by at least 50% within twelve weeks (measured by eczema area and severity index), versus 35% in the placebo group.
In 2016 good results were announced from the SOLO1 and SOLO2 phase III trials (for atopic dermatitis/eczema symptoms) and the US FDA granted it priority review status with a final decision due by March 2017.
