ICD-9-CM 00.55 | MeSH D054855 | |
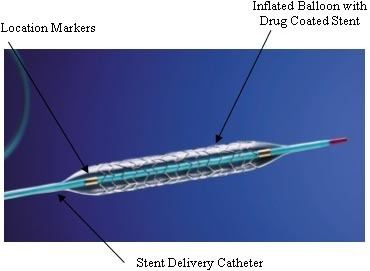 | ||
A drug-eluting stent (DES) is a peripheral or coronary stent (a scaffold) placed into narrowed, diseased peripheral or coronary arteries that slowly releases a drug to block cell proliferation. This prevents fibrosis that, together with clots (thrombi), could otherwise block the stented artery, a process called restenosis. The stent is usually placed within the peripheral or coronary artery by an interventional cardiologist or interventional radiologist during an angioplasty procedure.
Contents
- Medical uses
- Off label use
- Alternatives to stents in general
- Risks
- Stent thrombosis
- Design
- History
- Society and culture
- References
Drug-eluting stents in current clinical use were approved by the FDA after clinical trials showed they were statistically superior to bare-metal stents for the treatment of native coronary artery narrowings, having lower rates of major adverse cardiac events (usually defined as a composite clinical endpoint of death + myocardial infarction + repeat intervention because of restenosis). The first drug-eluting stents to be approved in Europe and the U.S. were coated with paclitaxel or an mTOR inhibitor, such as sirolimus.
Medical uses
Clinical trials have shown the benefits of coronary stenting with bare-metal stents over other methods of angioplasty, including balloon angioplasty and atherectomy. Drug-eluting stents (DES) have also been extensively studied, and are generally superior to bare-metal stents with respect to occurrence of major adverse cardiac events (MACE, generally defined as death, myocardial infarction, or the need for a repeat revascularization procedure). Stents are indicated to improve the diameter of the coronary artery lumen, when narrowing (generally because of atherosclerosis) causes ischemia (reduced oxygen delivery to the muscle supplied by that artery).
Off-label use
Drug-eluting stents also have been shown to be superior to bare-metal stents in reducing short-term complications of stenting in saphenous vein grafts; however, use in these bypass grafts is an example of an "off-label" use of drug-eluting stents. That is, this application has not been sufficiently examined by the Food and Drug Administration for that agency to recommend the use. For "on-label" applications, the FDA "believes that coronary drug-eluting stents remain safe and effective when used for the FDA-approved indications. These devices have significantly reduced the need for a second surgery to treat restenosis for thousands of patients each year."
Some concern has been expressed about overzealous use of stents in general. Two studies found about half of patients received stents for unapproved reasons, with worse outcomes for the patients in both studies. More recent data suggest off-label use of both bare-metal stents and drug-eluting stents have increased risks. However, drug-eluting stents seemed to have similar or improved rates of death or MI compared with bare-metal stents, and consistently reduced need for target vessel revascularization. Overall, the data support the use of drug-eluting stents for off-label indications.
Alternatives (to stents in general)
Medical therapy for coronary artery disease has also improved since the 1970s, and for many kinds of patients may be as successful as stenting or surgery. For those requiring PCI or surgery, medical therapy and revascularization should be viewed as complementary rather than opposing strategies.
Coronary artery bypass graft surgery is the best treatment for some patients. Differences between outcomes with stenting and with coronary artery bypass surgery (CABG) are a point of controversy. A recent study comparing the outcomes of all patients in New York state treated with CABG or percutaneous coronary intervention (PCI) demonstrated CABG was superior to PCI with DES in multiple vessel coronary artery disease . Patients treated with CABG had lower rates of death and of death or myocardial infarction than treatment with a drug-eluting stent. Patients undergoing CABG also had lower rates of repeat revascularization.
Two major randomized controlled trials comparing CABG and DES are either completed or ongoing, and have published results - Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) and Future Revascularization Evaluation in Patients With Diabetes Mellitus—Optimal Management of Multivessel Disease (FREEDOM). The five-year follow-up results of SYNTAX showed, depending on the complexity of coronary vessel disease, PCI was either equally effective or inferior to CABG. Similarly, results from the FREEDOM trial published after five years showed CABG to be superior to PCI in reducing rates of death and myocardial infarction. Both trials found either increased or insignificantly different rates of stroke with CABG as compared to PCI. The registries of the nonrandomized patients screened for these trials may provide as much robust data regarding revascularization outcomes as the randomized analysis.
Other studies, including the ARTS II registry, suggest drug-eluting stenting is not inferior to coronary bypass for treatment of multiple-vessel coronary disease. The ARTS II registry compared a cohort of patients treated with multiple-vessel stenting with DES, to the historical CABG cohort in the ARTS I trial (itself a randomized comparison between multiple-vessel bare-metal stenting vs. CABG.) At three-year follow-up, major adverse cardiac events were comparable between the ARTS II DES group and the ARTS I CABG group. Reintervention was lower in the ARTS I CABG group. In all comparison studies of stenting vs. bypass surgery, only a small minority of patients with multiple-vessel coronary disease have been eligible for inclusion in the studies, and for most patients, clinical judgement by experienced operators suggest one or the other approach is preferred.
Risks
Like all invasive medical procedures, implanting stents in the coronary arteries carries risk. For the newer drug-eluting stents, very-long-term results are not yet available; however, five years after implantation, sirolimus-eluting stents remained superior to bare-metal stents.
Risks associated with cardiac catheterization procedures include bleeding, allergic reaction to the X-ray contrast agents used to visualize the coronary arteries, and myocardial infarction. With PCI, the requirement for emergency CABG has markedly decreased since the days of balloon angioplasty, such that in some communities, coronary stenting is permitted in hospitals without on-site cardiac surgery facilities, though this remains highly controversial in the United States, not the least because of the rare but largely unpredictable risk of coronary artery perforation. Rarely, a type of allergic reaction to the drug may occur; episodes of fatality have been reported.
Stent thrombosis
Although drug-eluting stents continue to represent a major medical advance for angioplasty, evidence has always shown new clot thrombosis formation with stents to be a problem, thus clotting suppressant agents are routinely given during placement, and anticlotting agents should be continued; the question is for how long. Coronary arterial healing occurs after the placement of a drug-eluting stent, but complete healing of the vessel takes time. For drug-eluting stents, the time course of complete healing in humans is unknown.
A stent is a foreign object in the body, and the body responds to the stent’s presence in a variety of ways. Macrophages accumulate around the stent, and nearby smooth muscle cells proliferate. These physiological changes, which can cause restenosis, are limited by the drugs released by the stent, but these drugs also limit formation of a new endothelial layer over the new stent to inhibit clot formation. Endothelialization is a hallmark of vascular healing and is important for the prevention of thrombus formation. Lack of healing caused by antiproliferative drugs can make the stent an exposed surface on which a clot, sometimes life-threatening, can form. For drug-eluting stents (which, by design, delay formation of a new endothelium cover over the stent), the incidence of clot formation within the stent may persist for a longer period of time, perhaps as long as five years after treatment. Drug-eluting stents have been associated with delayed arterial healing and the prevalence of latent thrombus after five years, suggesting patients may continue to be at risk for stent thrombosis for an extended period of time.
Though less frequent with drug-eluting stents, neointimal proliferation can still occur in DES and cause restenosis. Stent occlusion because of thrombosis may occur during the procedure, in the following days, or later. The presence of thrombi around the stent may, in turn, affect the drug-eluting performance of the stent. Treatment with the antiplatelet drugs aspirin and clopidogrel appears to be the most important factor reducing this risk of thrombosis, and early cessation of one or both of these drugs after drug-eluting stenting markedly increases the risk of stent thrombosis and myocardial infarction. A recent histopathology study showed very late DES thrombosis is associated with histopathological signs of inflammation and intravascular ultrasound evidence of vessel remodeling. Compared with other causes of myocardial infarction, eosinophilic infiltrates are more common in thrombi harvested from very late DES thrombosis and correlate with the extent of stent malapposition.
Whether drug-eluting stents are at higher risk than bare-metal stents for late thrombosis is intensely debated. In meta-analyses of the sirolimus- and paclitaxel-eluting stent trials, a small but statistically higher risk of thrombosis was shown after the first year, compared to bare-metal stents. Late stent thrombosis often causes myocardial infarction and sometimes death. In other analyses, the late thrombosis risk is offset by drug-eluting stents' markedly reduced risk of restenosis and its complications including myocardial infarction. A meta-analysis concluded the mortality risk associated with drug-eluting and bare-metal stents is similar.
Design
Drug-eluting stents generally consist of three parts - the stent platform, a polymer coating that binds the drug to the stent and releases drug (although stents have been tested that do without a coating), and the drug.
The stent platform itself is an expandable framework, generally with an elaborate mesh-like design to allow expansion, flexibility, and in some cases the ability to make/enlarge side openings for side vessels. The first DES were stainless steel alloys composed of iron, nickel, and chromium and were based on existing bare metal stents. These stents were hard to visualize with medical imaging, posed a risk of causing allergic responses, and were difficult to deliver, and subsequent new alloys were brought to bear, namely cobalt-chrome and platinum chrome, with improved performance. Subsequently, bioresorbable stents have been developed in which the stent itself dissolves over time. As of 2009, materials that had been explored included magnesium, polylactic acid, polycarbonate poylmers, and salicylic acid polymers. Resorbable stents have held the promise of providing an acute treatment that would eventually allow the vessel to function normally, without leaving a permanent device behind.
One to three or more layers of polymer can be used in the coating, e.g., a base layer for adhesion, a main layer that holds and elutes (releases) the drug into the arterial wall by contact transfer, and sometimes a top coat to slow down the release of the drug and extend its effect. The first few drug-eluting stents to be licensed used durable coatings. The first generation coatings appear to have caused immunological reactions at times and some possibly led to thrombosis, which has driven experimentation and development of new coating approaches.
The drug is mainly to inhibit neointimal growth (due to proliferation of smooth muscle cells) which would cause restenosis. Much of the neointimal hyperplasia seems to be caused by inflammation. Hence, immunosuppressive and antiproliferative drugs are used. Sirolimus, paclitaxel, and everolimus were previously used for other medical applications and have been included in licensed DES.
History
The first procedure to treat blocked coronary arteries was coronary artery bypass graft surgery (CABG), wherein a section of vein or artery from elsewhere in the body is used to bypass the diseased segment of coronary artery. In 1977, Andreas Grüntzig introduced percutaneous transluminal coronary angioplasty (PTCA), also called balloon angioplasty, in which a catheter was introduced through a peripheral artery and a balloon expanded to dilate the narrowed segment of artery. As equipment and techniques improved, the use of PTCA rapidly increased, and by the mid-1980s, PTCA and CABG were being performed at equivalent rates. Balloon angioplasty was generally effective and safe, but restenosis was frequent, occurring in about 30–40% of cases, usually within the first year after dilation. In about 3% of balloon angioplasty cases, failure of the dilation and acute or threatened closure of the coronary artery (often because of dissection) prompted emergency CABGs.
Dotter and Melvin Judkins had suggested using prosthetic devices inside arteries (in the leg) to maintain blood flow after dilation as early as 1964. In 1986, Puel and Sigwart implanted the first coronary stent in a human patient. Several trials in the 1990s showed the superiority of stent placement over balloon angioplasty. Restenosis was reduced because the stent acted as a scaffold to hold open the dilated segment of artery; acute closure of the coronary artery (and the requirement for emergency CABG) was reduced, because the stent repaired dissections of the arterial wall. By 1999, stents were used in 84% of percutaneous coronary interventions (i.e., those done via a catheter, and not by open-chest surgery).
Early difficulties with coronary stents included a risk of early thrombosis (clotting) resulting in occlusion of the stent. Coating stainless steel stents with other substances such as platinum or gold did not eliminate this problem. High-pressure balloon expansion of the stent to ensure its full apposition to the arterial wall, combined with drug therapy using aspirin and another inhibitor of platelet aggregation (usually ticlopidine or clopidogrel) nearly eliminated this risk of early stent thrombosis.
Though it occurred less frequently than with balloon angioplasty or other techniques, stents nonetheless remained vulnerable to restenosis, caused almost exclusively by neointimal tissue growth. To address this issue, developers of drug-eluting stents used the devices themselves as a tool for delivering medication directly to the arterial wall. While initial efforts were unsuccessful, the release (elution) of drugs with certain specific physicochemical properties from the stent was shown in 2001 to achieve high concentrations of the drug locally, directly at the target lesion, with minimal systemic side effects. As currently used in clinical practice, "drug-eluting" stents refers to metal stents that elute a drug designed to limit the growth of neointimal scar tissue, thus reducing the likelihood of stent restenosis.
The first successful trials were of sirolimus-eluting stents. A clinical trial in 2002 led to approval of the sirolimus-eluting Cypher stent in Europe in 2002. After a larger pivotal trial (one designed for the purpose of achieving FDA approval), published in 2003, the device received FDA approval and was released in the U.S. in 2003. Soon thereafter, a series of trials of paclitaxel-eluting stents led to FDA approval of the Taxus stent in 2004.
The first resorbable stent tested in humans was developed by the Igaki Medical Planning Company in Japan and was constructed from poly-L-lactic acid (a form of polylactic acid); they published their initial results in 2000. The German company, Biotronik, developed a magnesium absorbable stent and published clinical results in 2007. The first company to bring a bioresorbable stent to market was Abbott Vascular which received a European marketing approval in September 2012; the second was Elixir which received its CE mark in May 2013.
Society and culture
In 2012, a meta-analysis of clinical trial data was published, showing that, for people with stable coronary artery disease, DES has no benefit compared to treatment with drugs. The New York Times interviewed the study's main author, who said that more than half of patients with stable coronary artery disease were implanted with stents without even trying drug treatment and that he believed this happened because hospitals and doctors wanted to make more money. In 2013 the Times of India reported that DES were widely overused and that Indian distributors used profits from high markups on DES to bribe doctors to use them. In 2014 an investigation by the Maharashtra Food and Drug Administration found that high markups and bribery related to DES was still widespread.
