 | ||
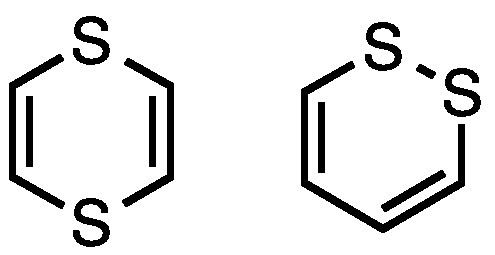
Dithiin is a class of heterocyclic compounds, with the parent members having the formula (C2H2)2S2. Two isomers of this parent are recognized, 1,2- and 1,4-dithiins. These compounds are antiaromatic consisting of 8π e− systems, akin to cyclooctatetraene. Vinyldithiin, a common component of garlic, is a misnomer for 3-vinyl-4H-1,2-dithiin, since true 1,3-dithiins are unknown.
Contents
1,4-Dithiins
1,4-Dithiins have been more extensively studied. They are usually prepared by condensation of the equivalent of α-mercaptocarbonyls. For example, the acetal HSCH2CH(OEt)2 converts upon heating to the parent 1,4-dithiin. Being antiaromatic, they are nonplanar and can be oxidized to their radical cations. Photolysis leads to dimerization via a [2+2] cycloaddition. Thianthrene is dibenzo-1,4-dithiin.
1,2-Dithiins
1,2-Dithiins are isomers of but-2-ene-dithials. They tend to be unstable with respect to loss of sulfur and formation of the thiophene derivative:
C4R4S2 → C4R4S + "S"They are often claret-colored. Some occur as flower pigments in plants of the asteraceae family.
