Formula S2N2 Appearance colourless crystals | Molar mass 92.1444 g/mol | |
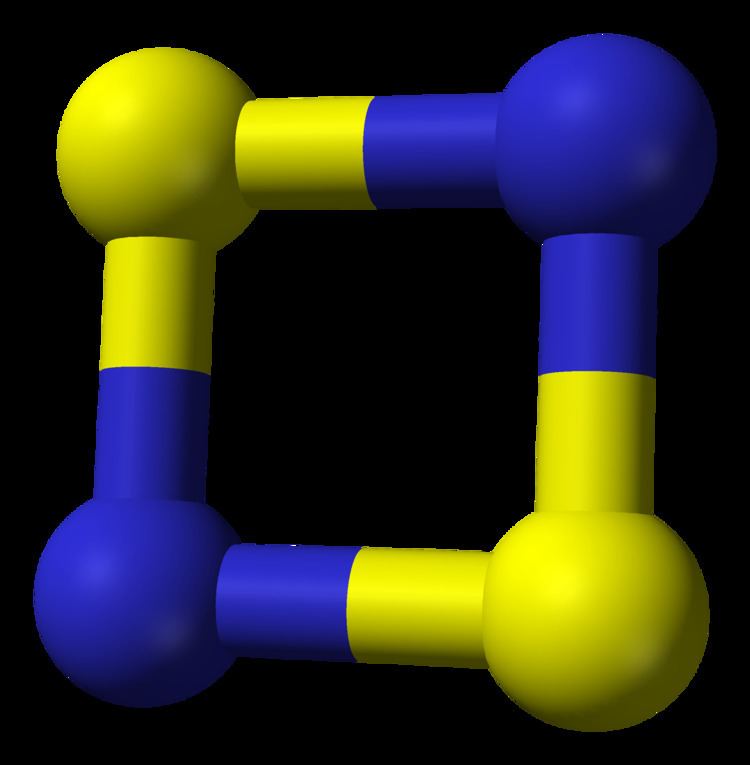 | ||
Disulfur dinitride is the chemical compound S2N2 with a cyclic square planar structure.
Contents
Preparation and reactions
Passing gaseous S4N4 over silver metal wool at 250–300 °C at low pressure (1mm Hg) yields cyclic S2N2. The silver reacts with the sulfur produced by the thermal decomposition of the S4N4 to form Ag2S, and the resulting Ag2S catalyzes the conversion of the S4N4 into the four-membered ring S2N2,
S4N4 + 8 Ag → 4 Ag2S + 2 N2S4N4 → 2S2N2An alternative uses the less explosive S4N3Cl.
S2N2 decomposes explosively above 30°C, and is shock sensitive. It readily sublimes, and is soluble in diethyl ether. Traces of water cause it to polymerize into S4N4. In the solid state it spontaneously polymerizes forming (SN)x. It forms adducts with Lewis acids via a nitrogen atom, e.g. S2N2 · BCl3, S2N2 · 2AlCl3, S2N2 · SbCl5, S2N2 · 2SbCl5.
Structure and bonding
The S2N2 molecule is virtually square and planar. The S-N bond lengths are 165.1pm and 165.7pm and the bond angles are very close to 90°. The S2N2 molecule is isoelectronic with the S42+ dication and has 6π electrons. The bonding has been investigated using a spin-coupled valence bond method and is described as having four framework sigma bonds, with the N atoms bearing a high negative charge and the S atoms a corresponding positive charge. Two π electrons from the sulfur atoms are coupled across the ring making the molecule overall a singlet diradical.
